Figure 4.
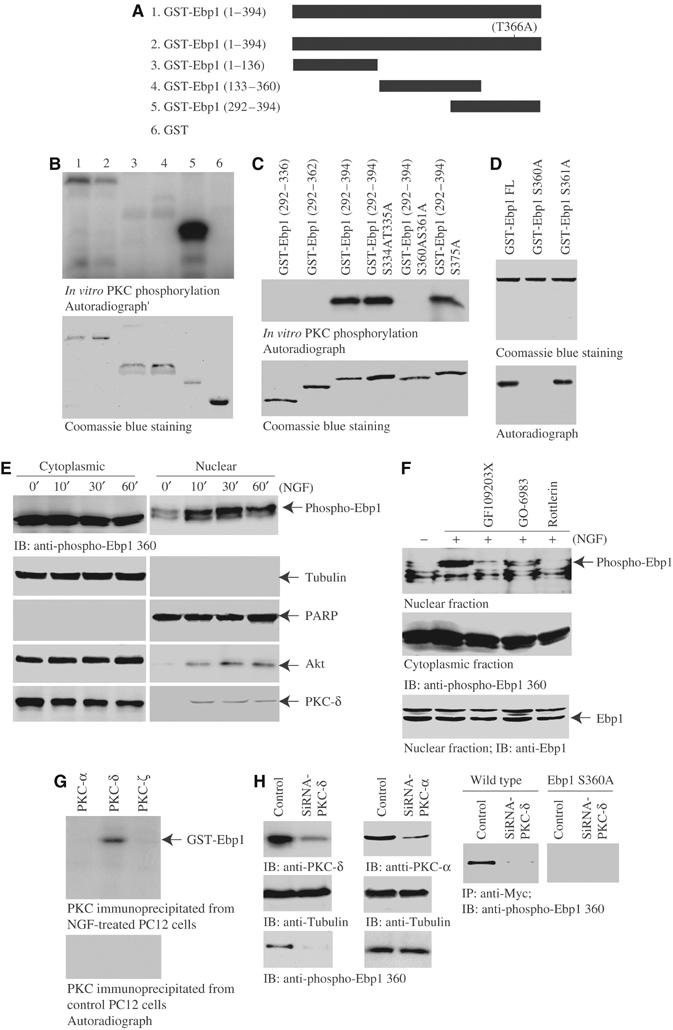
PKC phosphorylates Ebp1 on serine 360. (A) Diagram of various GST-Ebp1 fragments. (B) In vitro PKC assay. In total, 2 μg of purified GST-Ebp1 fusion proteins was incubated with active PKC in the presence of γ-32P-ATP. Both wild-type Ebp1 and T366A mutant were strongly phosphorylated. Interestingly, fragment 292–394 was strongly phosphorylated (upper panel). The identity of purified recombinant proteins were verified by Coomassie blue staining (lower panel). (C) In vitro PKC assay with C-terminal fragments of Ebp1. S360 and 361 might be the phosphorylation sites (upper panel). Coomassie blue staining of GST-Ebp1 fragments (lower panel). (D) In vitro PKC assay with Ebp1 mutants. S360A mutation disrupts PKC phosphorylation (lower panel). Coomassie blue staining of GST-Ebp1 mutants (upper panel). (E) PKC phosphorylates Ebp1 in cells. PC12 cells were treated with NGF for various times, and the cytoplasmic and nuclear fractions were prepared. NGF stimulation does not significantly alter the cytoplasmic Ebp1 phosphorylation. By contrast, NGF markedly provokes nuclear Ebp1 phosphorylation at 30 min. The purity and identity of each fraction was confirmed by immunoblotting with anti-tubulin and anti-PARP antibodies (second and third panels). NGF provokes both Akt and PKC-δ nuclear translocation (fourth and bottom panels). (F) PKC inhibitors block NGF-elicited Ebp1 phosphorylation in the nucleus. PC12 cells were pretreated with various PKC inhibitors for 30 min, followed by NGF for 30 min, and the cytoplasmic and nuclear fractions were prepared. NGF-provoked nuclear Ebp1 phosphorylation was inhibited by 10 μM GF109203X and 60 nM Go6983, and completely abrogated by 6 μM Rotterlin. By contrast, cytoplasmic Ebp1 phosphorylation is not diminished. The total Ebp1 in the nuclear fraction is verified by immunoblotting (bottom panel). (G) In vitro kinase assay with PKC isoform proteins. GST-Ebp1 (1 μg) and [γ-32P]ATP were incubated with various PKC isoforms at 30°C for 1 h. PKC isoforms were immunoprecipitated from NGF-treated or control PC12 cells. Compared to control, NGF-treated PKC-δ but not -α or -ζ selectively phosphorylates Ebp1. (H) Knocking down of PKC-δ abolishes Ebp1 phosphorylation. PC12 cells were transfected with PKC-δ and -α siRNA, respectively. In 48 h, the cells were stimulated with NGF. Both PKC isoforms were substantially removed upon transfection of their siRNA (left top panel). Depletion of PKC-δ but not PKC-α blocks the phosphorylation of nuclear Ebp1 (left bottom panels). Myc-tagged wild-type Ebp1 and S360A stably transfected PC12 cells were transfected with PKC-δ siRNA. In 48 h, the cells were stimulated with NGF, Ebp1 was immunoprecipitated from nuclear fraction with anti-Myc antibody. Potent Ebp1 phosphorylation was revealed in wild-type Myc-Ebp1 stably transfected cells, which was completely diminished in PKC-δ-knocked down cells. By contrast, no phosphorylation was detected in Myc-Ebp1S360A-transfected cells (right panels).
