Figure 4.
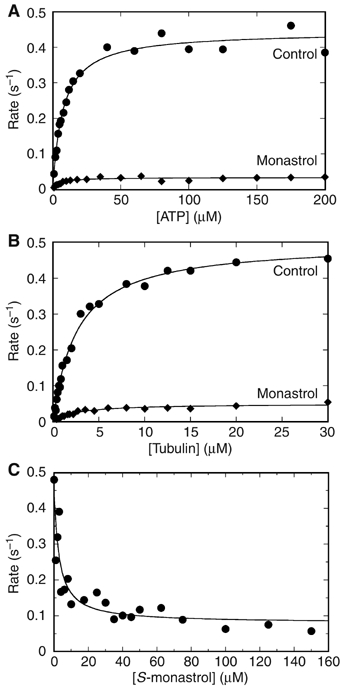
Steady-state ATPase kinetics and monastrol inhibition. A preformed MT·Eg5 (•) or MT·Eg5-monastrol (⧫) complex was rapidly mixed with [α-32P]ATP. (A) Final concentrations: 0.5 μM Eg5-513 motor domain, 20 μM tubulin, 20 μM Taxol, 150 μM S-monastrol, and 1–200 μM MgATP. The fit of the data to the Michaelis–Menten equation provided the steady-state parameters: kcat=0.44±0.01 s−1, Km,ATP=7.5±0.7 μM and kcat-Mon=0.034±0.001 s−1, Km,ATP-Mon=4.5±0.9 μM. (B) Final concentrations: 1 μM Eg5-513 motor domains, 0.1–40 μM tubulin, 20 μM Taxol, 150 μM S-monastrol, and 400 μM MgATP. The data were fit to equation (1): kcat=0.48±0.01 s−1, K1/2,MT=1.8±0.1 μM and kcat-Mon=0.048±0.002 s−1, K1/2,MT -Mon=1.7±0.3 μM. (C) Final concentrations: 0.5 μM Eg5-513 motor domain, 20 μM tubulin, 20 μM Taxol, 150 μM MgATP, and 0–150 μM S-monastrol. The data were fit to equation (2): Kd-Mon=2.88±0.04 μM.
