Figure 7.
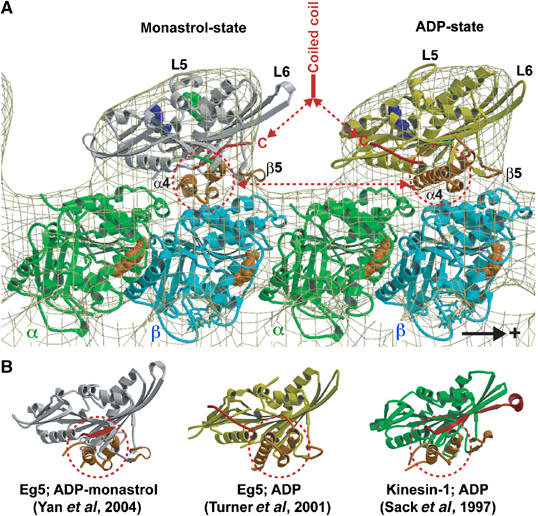
Molecular docking of the atomic resolution X-ray structures of monomeric Eg5 in the presence of ADP and monastrol (left; Yan et al, 2004) and ADP (right; Turner et al, 2001) into our EM-derived 3-D map of the MT·Eg5-513·AMPPNP complex. The plus-end of the MT is on the right. One major structural difference in the two heads that is relevant to MT binding is the position of helix α4 that is rotated by about 20° from the monastrol to ADP structure. Monastrol (green in (A)) binds close to the ATP pocket. The other relevant feature is the locked neck linker (red) in the monastrol structure. Hence, the arrangement as shown here could mimic a dimer with the trailing head in an ATP state and the leading head ready to release ADP and assume a nucleotide-free state. (B) Crystal structures of monomeric Eg5 in the presence of monastrol and ADP, Eg5 with ADP, and rat kinesin with ADP displayed in aligned orientations according to their internal β-sheet.
