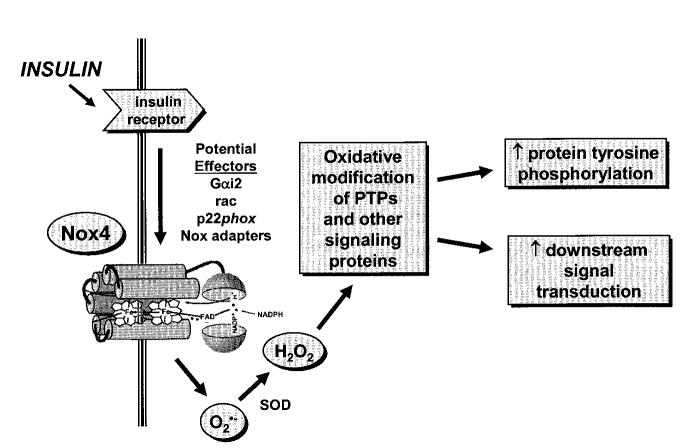
FIG. 2.
Postulated effectors of insulin-induced ROS via Nox4 and influences on downstream events in the insulin action cascade. Insulin stimulation of its target cells, especially adipocytes, elicits a burst of superoxide, with rapid generation of H2O2 catalyzed by cellular superoxide dismutase (SOD). As discussed in the text, the Nox homolog Nox4 may mediate a major part of the insulin-induced ROS generation in adipocytes (98). However, the mechanism(s) coupling Nox4 with the insulin receptor is not known. Potential effectors of the insulin activation of Nox activity may include the G-proteins rac and Gαi2, the flavocytochrome b558 subunit p22phox, or interactions with novel Nox adaptor proteins (perhaps homologous to NOXO1 [Nox organizer 1] or NOXA1 [Nox activator 1]), among other possibilities. Insulin-stimulated ROS are believed to interact with a limited set of cellular proteins that contain catalytic thiol side-chains known to be particularly susceptible to biochemical oxidation (see Table 1). Inhibition of these signaling proteins, e.g., PTPs, leads to enhanced tyrosine autophosphorylation of the receptor and its substrate proteins as well as alterations in downstream signaling in the insulin action cascade. See text for further discussion and references.