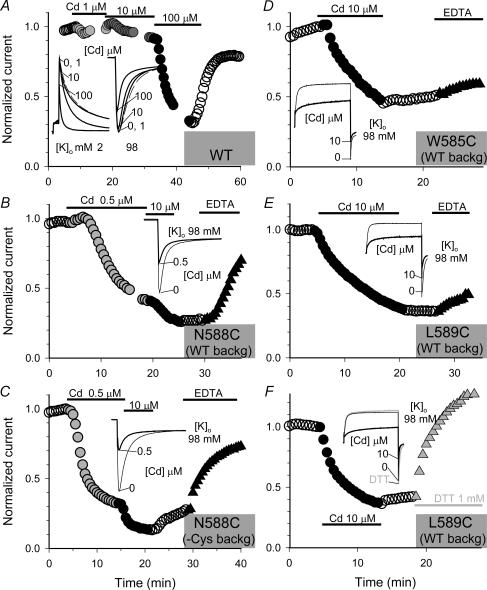
Figure 6. Coordination of high-affinity Cd2+ binding by cysteine side chains introduced into positions 585, 588 and 589, but not by native cysteines in WT hERG.
Shown in the main graphs are time courses of changes in current amplitude of WT hERG (A), N588C in WT- or Cys-removed background (B and C), W585C and L589C in WT-background (D–F). Channel types are denoted in grey shading areas. Currents were recoded before, during and after exposure to Cd2+ of specified concentrations (marked above or below data points) and, for cysteine-substituted mutants, during exposure to 1 mm EDTA (B–E) or 1 mm DTT (F). Oocytes expressing cysteine-substituted mutants were pretreated with DTT, and Cd2+ effects were detected after extensive washout of DTT. For cysteine-substituted mutants, recordings were made in 98 mm[K+]o (marked in inset). For WT hERG, the main graph and the left part of the inset were recorded in 2 mm[K+]o while the right part of the inset was recorded in 98 mm[K+]o (marked in inset). Currents were elicited by 1-s depolarization pulses to +60 mV followed by repolarization to –60 mV (WT, in 2 mm[K+]o) or –120 mV (WT, in 98 mm[K+]o, all cysteine-substituted mutants, in 98 mm[K+]o). Current amplitudes were quantified by the peak of tail currents and normalized by the control current amplitude in each experiment. Insets: selected current traces from the same experiments as shown in the main graphs. Shown are either tail currents only (WT in 98 mm[K+]o (A), and N588C (B–C) or both test pulse currents and tail currents.