Abstract
The present study was designed to examine (1) whether there are vasodilator fibres in the masseter muscle, and (2) if there are, to establish the neural pathways mediating these responses in urethane-anaesthetized rats. Electrical stimulation of the central cut end of the lingual nerve (LN) elicited intensity- and frequency-dependent increases of the blood flow in the masseter muscle (MBF) and lower lip (LBF). Increases in both the MBF and LBF evoked by the LN stimulation were reduced by hexamethonium in a dose-dependent manner (1–10 mg kg−1). Pretreatment with phentolamine or propranolol at a dose of 100 μg kg−1 had no effect on the increases in either MBF or LBF evoked by LN stimulation. Pretreatment with atropine (100 μg kg−1) significantly reduced the MBF increase induced by LN stimulation, but not that in the LBF. The sectioning of the superior cervical sympathetic trunk did not affect the responses. MBF increases occurred with electrical stimulation of the trigeminal ganglion, and these increases were significantly reduced by the administration of hexamethonium and atropine. Lidocaine microinjection into the trigeminal spinal nucleus or salivatory nuclei caused a significant attenuation of the LN-induced MBF increases. When wheat germ agglutinin–horseradish peroxidase (WGA–HRP) was injected into the masseter muscle, labelled neurones were abundantly observed in the otic ganglion. The present study indicates that there are parasympathetic cholinergic and noncholinergic vasodilator fibres originating from cell bodies in the otic ganglion in the rat masseter muscle. The MBF increase evoked by activation of the parasympathetic fibres occurred via the trigeminal mediated reflex, suggesting that the novel parasympathetic vasodilator response may play an important role in the regulation of the haemodynamics of jaw muscles.
The blood flow in skeletal muscle is generally considered to be regulated by local tissue factors (oxygen concentration, potassium ions, acetylcholine, adenosine triphosphate, lactic acid and carbon dioxide) and neural mechanisms (the sympathetic nervous system) (Guyton & Hall, 1996). Details of the regulatory mechanism of the blood flow in active skeletal muscles are poorly understood, but a neural mechanism is particularly appealing because of the rapidity of increases in skeletal muscle blood flow.
Skeletal muscles are well known to be provided with sympathetic vasoconstrictor nerves and, in some species of animals, sympathetic vasodilator nerves. The sympathetic vasoconstrictor fibres secrete noradrenaline, which acts on the muscle vessels to cause the vasoconstrictor effect by α–adrenoceptors (Joyner & Halliwill, 2000; Thomas & Segal, 2004). In cats and some other animals, there are also sympathetic cholinergic vasodilator fibres (Bolme et al. 1970). These could produce a specific and targeted vasodilatation that would facilitate blood flow and oxygen delivery to skeletal muscle in anticipation of the metabolic demands associated with exercise (Joyner & Halliwill, 2000), but such fibres have not been proven to be present in humans.
It is generally considered that vasodilatation and vasoconstriction are basic physiological adjustments to the metabolic demands of skeletal muscles, and disturbances in intramuscular blood flow can be related to muscle pain (Rasmussen et al. 1977; Lund et al. 1986). Hidaka et al. (2004) recently reported that the haemodynamics of jaw muscles are susceptible to mental stress, suggesting a role in the aetiology of jaw muscle dysfunction during mental stress. They showed notable changes in haemodynamic parameters of the masseter muscle under mental stress associated with increases in sympathetic nervous activity, despite only small changes in the integrated electromyographic activity. Individuals with a history of chronic jaw muscle pain linked to dysfunction of the sympathetic nervous system have slow intramuscular reperfusion during the recovery phase after sustained isometric contractions (Delcanho et al. 1996). Nervous control of the blood flow in the jaw muscles (jaw opening and closing muscles), especially in the masseter muscle, has been investigated by some workers (Alm, 1975; Granstam & Nilsson, 1990; Matsuo et al. 1995). Electrical stimulation of sympathetic nerves derived from the superior cervical sympathetic trunk (CST) has been reported to induce vasoconstrictor responses in the masseter muscles of the monkey (Alm, 1975), rabbit (Granstam & Nilsson, 1990) and rat (Matsuo et al. 1995), which are completely suppressed by α–adrenoceptor blockage (Granstam & Nilsson, 1990). However, there are no reports of the vasodilator response in the masseter muscle under physiological conditions.
The present study was thus designed to examine (1) whether there are vasodilator fibres in the masseter muscle, and (2) if there are, to establish the neural pathways mediating these responses in deeply urethane-anaesthetized, artificially ventilated rats.
Methods
Preparation of animals
Experiments were performed on adult male Wistar rats between 15 and 30 weeks of age and weighing 280–480 g. After induction with inhalation anaesthesia (ether), urethane (1 g kg−1) in a volume of 1 ml (100 g body weight)−1 was subcutaneously injected into the back of the animals. One femoral vein was cannulated to allow drug injection, and one femoral artery was cannulated and connected to a Statham pressure transducer to monitor the systemic arterial blood pressure (SABP) and heart rate. The anaesthetized animals were intubated, paralysed by intravenous (i.v.) injection of pancuronium bromide (Mioblock; Organon Teknika, Netherlands; 0.6 mg kg−1 initially, supplemented with 0.4 mg kg−1 every hour or so after testing the level of anaesthesia; see below), and artificially ventilated via the tracheal cannula with a mixture of 50% air–50% O2. The ventilator (model SN-480-7; Shinano, Tokyo, Japan) was set to deliver a tidal volume of 5–7.5 cm3 kg−1 at a rate of 70 breaths min−1, and the end-tidal concentration of CO2 was determined by means of an infrared analyser (Capnomac Ultima; Datex, Helsinki, Finland), as reported elsewhere (Izumi & Karita, 1990). Rectal temperature was maintained at 37–38°C with the use of a heating pad. Before the injection of further pancuronium bromide, we checked that depth of anaesthesia was adequate by the absence of a flexion response to a noxious stimulus, such as pinching the digit for approximately 2 s. When the depth of anaesthesia was considered inadequate, additional urethane (i.e. intermittent doses of 100 mg kg−1, i.v.) was administered.
At the end of the experiment, all the rats were killed by an overdose of pentobarbital sodium. The experimental protocols were conducted in accordance with the Guidelines for Animal Experiments issued by the Health Sciences University of Hokkaido. All the animals were cared for in accordance with the recommendations in the current National Research Council guide.
Electrical stimulation of the lingual nerve and superior CST
The central cut end of the lingual nerve (LN) (a in Fig. 1) was electrically stimulated using a bipolar silver electrode attached to an electrical stimulator (model SEN-7103; Nihon Kohden, Tokyo, Japan). For this purpose, the LN was sectioned and stimulated unilaterally under a binocular microscope. The LN was stimulated for 20 s with various voltages (1–30 V) at various frequencies (1–50 Hz) using 2 ms pulse durations. In some experiments, the peripheral cut end of the superior CST (c in Fig. 1) was briefly stimulated for 20 s with 10 V at 10 Hz using 2 ms pulses. In all experiments, the cervical vagi and CST were cut bilaterally in the neck before the stimulation, unless otherwise noted. This ensured that only nonvagal parasympathetic effects were involved in the present study.
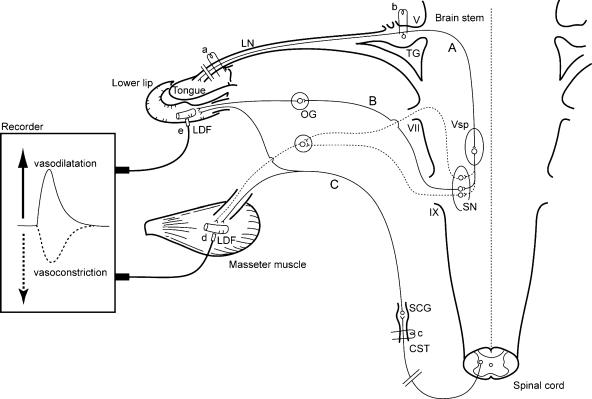
Figure 1. Schematic representation of the sites of electrical stimulation and blood flow measurements in the rats.
Stimulation sites: central cut end of the lingual nerve (LN) (a), trigeminal ganglion (TG) (b), and peripheral cut end of superior cervical sympathetic trunk (CST) (c). Blood flow measurement sites: masseter muscle (d) and lower lip (e) by laser-Doppler flowmeter (LDF). The continuous lines indicate trigeminal sensory inputs to the brain stem (A), the parasympathetic vasodilator fibres to the lower lip from the salivatory nuclei (SN) (B) (Kaji et al. 1991), and the sympathetic vasoconstrictor fibres to both masseter muscle and lower lip from the CST (C). The dashed lines indicate the possible pathways by which nerve excitation may evoke vasodilatation in the masseter muscle in response to LN stimulation. OG, otic ganglion; SCG, superior cervical ganglion; Vsp, trigeminal spinal nucleus; V, trigeminal nerve root; VII, facial nerve root; and IX, glossopharyngeal nerve root.
Stimulation of the trigeminal ganglion
The animals were mounted in a stereotaxic frame (Narishige, Tokyo, Japan), and a concentric bipolar electrode (Inter Medical, Tokyo, Japan), insulated with enamel except at the tip, was positioned within the trigeminal ganglion (TG) (b in Fig. 1) via a small burr hole in the skull. The electrode was advanced by means of a micromanipulator to the intended location, which was at stereotaxic coordinates 4 mm rostral to the interaural line, and 3.5 mm lateral to the midline. In each penetration, the electrode was advanced carefully until it made contact with that portion of the skull surface (alisphenoid) that lies beneath the TG, after which it was withdrawn 0.5 mm, to place it within the TG (since the TG is approximately 1 mm in depth; Paxinos & Watson, 1998). We routinely used a 20 s train of rectangular pulses generated by a stimulator through the isolation unit mentioned above, usually with an amplitude of 20 V and a duration of 2 ms, at a frequency of 20 Hz. Reference sites were marked by passing a 1 mA current for 60 s after the injection of an overdose of pentobarbital sodium (see above). The location of the stimulation site was ascertained with the binocular microscope after an extensive craniectomy and excision of the brain rostral to the colliculi.
Central microinjection study with lidocaine
To determine whether the vasodilator response evoked by LN stimulation was mediated via the trigeminal spinal nucleus (Vsp) or salivatory nuclei (SN) as shown in Fig. 1, lidocaine (lignocaine; 4%) was microinjected into the Vsp or SN in a volume of 0.3 μl per site via an injection cannula (0.3 mm o.d.) inserted through an implanted guide cannula. The animal was mounted in a stereotaxic frame, as mentioned above, and after partial craniotomy, part of the tentorium cerebelli was removed by drilling. A guide cannula (0.6 mm o.d.) was positioned within the Vsp, which was at stereotaxic coordinates 2.6–4.8 mm ventral to the interaural line, 3–3.5 mm lateral to the midline, and 1–1.5 mm high to the interaural line, or the SN at stereotaxic coordinates 1.5–2.6 mm ventral to the interaural line, 1.8–2.2 mm lateral to the midline, and 1–1.5 mm high to the interaural line (Paxinos & Watson, 1998), via a small burr hole in the skull. All animals were given an overdose of pentobarbital sodium (see above) and perfused through the ascending aorta with 200 ml saline (0.9%), followed immediately by 500 ml of 10% formalin. Then, the brain stem and upper cervical spinal cord were removed and stored for 1–3 days in buffered 30% sucrose. After storage, 50-μm-thick sections were cut on a freezing microtome (HM 430; Carl Zeiss, Jena, Germany) and collected in 0.1 m phosphate buffer (pH 7.4). Sections were mounted on gelatin-coated slides and stained with thionin. Transmitted light was employed to identify microinjection sites.
Measurement of the blood flow and SABP
Changes in the blood flow in the masseter muscle (MBF; d in Fig. 1), lower lip (LBF; e in Fig. 1) and biceps femoris muscle (BBF) were monitored on both sides using a laser-Doppler flowmeter (LDF) (FLO-C1; Omegawave, Tokyo, Japan), as described elsewhere (Izumi & Karita, 1990, 1992, 1993; Izumi & Ito, 1998; Mizuta et al. 2000; Mizuta & Izumi, 2004). The probes were placed against the masseter muscle after making an incision in the cheek skin and lower lip without exerting pressure on the tissues. The masseter muscle was ascertained by the naked eye. The LDF values obtained in this way represent the blood flow in the superficial vessels in each tissue (Edwall et al. 1987; Kim et al. 1990). Electrical calibration for zero blood flow was performed for all recordings. Several gain levels could be selected, and the maximum output of a particular gain level (defined electrically) was set as 100%. The analog output of the equipment does not give absolute values, but shows relative changes in blood flow (for technical details and an evaluation of the LDF method see Stern et al. 1977). The output from the devices was continuously displayed on an eight-channel chart recorder (model W5000; Graphtec, Tokyo, Japan) at a speed of 10 mm min−1. The blood flow changes were assessed by measuring the height of the response. The SABP was recorded from the femoral catheter via a Statham pressure transducer. A tachograph (model AT-610G; Nihon Kohden, Tokyo, Japan) triggered by the arterial pulse was used to monitor the heart rate.
Pharmacological agents
To examine whether the reflexly evoked vasodilator responses were mediated via activation of the autonomic nervous system, hexamethonium (C6), an autonomic ganglion cholinergic blocker, was administered (1 or 10 mg kg−1, i.v.), and the stimulation was repeated 10 min later. To determine whether the vasodilator response was mediated via activation of α- and β-adrenoceptors or muscarinic receptors, the LN was stimulated before and after i.v. administration of phentolamine, propranolol or atropine, at a dose of 100 μg kg−1. The magnitude of the response obtained after each agent was expressed as a percentage of the control response recorded before its administration (mean ± s.e.m.).
Axoplasmic transport study
The rats were anaesthetized by intraperitoneal injection of pentobarbital sodium solution (50 mg kg−1) and laid in the lateral horizontal position. A small incision was made in the cheek skin to expose the surface of the masseter muscle. A 10 μl volume of 5% wheat germ agglutinin–horseradish peroxidase (WGA–HRP; Toyobo, Osaka, Japan) in sterile saline was injected into the prescribed locus in the anterior superficial part of the masseter muscle with a Hamilton microsyringe at a speed of 1 μl min−1. The needle was vertically inserted in the position between the facial nerve and parotid duct in the masseter muscle. After the injection, the needle was left in situ for 2 min to avoid backflow of the injected WGA–HRP solution. After a postinjection period of 48–72 h, the rats were reanaesthetized deeply and perfused with 200 ml of saline, followed by 500 ml of 25% formalin and 1.25% glutaraldehyde in 0.1 m sodium phosphate buffer at pH 7.4. The otic ganglion (OG), pterygopalatine ganglion (PPG), superior cervical ganglion (SCG), TG and brain stem were removed and, in cold buffer containing 30% sucrose, 40- or 50-μm-thick sections were cut on a freezing microtome, as described above. All sections were prepared for peroxidase histochemistry using the protocol of Kuchiiwa & Kuchiiwa (1996) who reported the modified tetramethylbenzidine reaction method (Mesulam, 1978). Transmitted light was employed to identify WGA–HRP-labelled cells.
Statistical analysis
All numerical data are given as means ± s.e.m. The statistical significance of changes in the test responses was assessed using analysis of variance (ANOVA) followed by a contrast test and a post hoc test (Fischer's PLSD). Differences were considered significant at P < 0.05. Data were analysed using a Macintosh computer with StatView 5.0 and Super ANOVA.
Results
Effect of the LN stimulation on the MBF, LBF and SABP
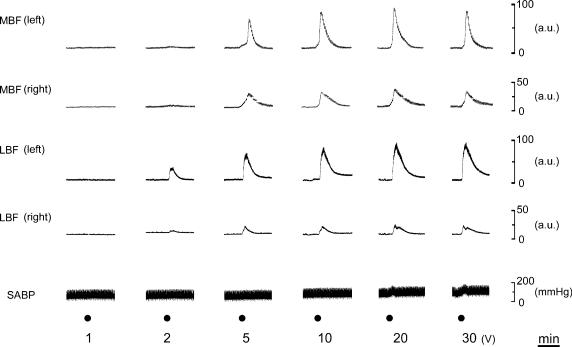
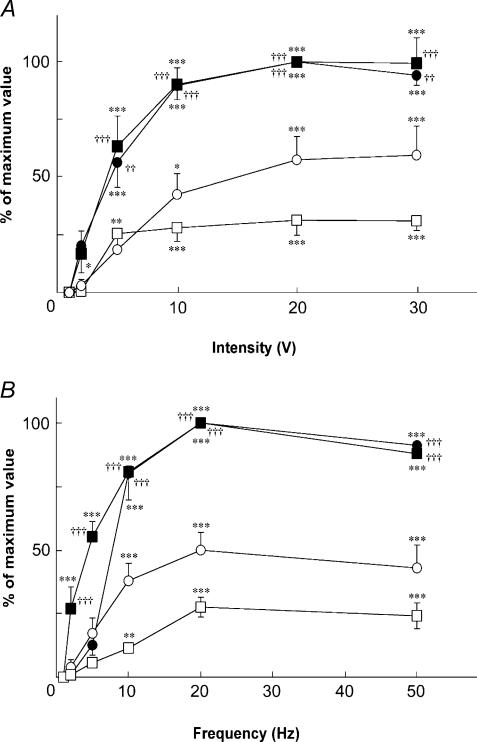
Figure 2 shows the changes evoked in the MBF, LBF and SABP following left LN stimulation for 20 s with various stimulus intensities (1–30 V) at 20 Hz, using pulse durations of 2 ms. This frequency was chosen because the optimal stimulus frequency for the LBF increase was around 20 Hz, as described elsewhere (Koeda et al. 2003). Intensity– and frequency–response curves were generated using stimulus trains for LN at 20 Hz, and at 20 V, respectively. Maximal increases in both MBF and LBF in response to LN stimulation were taken as 100%. Figure 3 shows mean data (± s.e.m.) for the effects of LN stimulation on both MBF and LBF when the LN stimulation was delivered at various intensities (1–30 V; Fig. 3A) and at various frequencies (1–50 Hz; Fig. 3B). Significant increases in both MBF and LBF on the left side took place above 5 and 2 V, respectively, (for MBF, F5,30 = 28.93, n = 6 in each group, P < 0.0001; for LBF, F5,30 = 28.97, n = 6 in each group, P < 0.0001). On the right side, LN stimulation also had a significant effect on the increases in both the MBF and LBF above 10 and 5 V, respectively, (for MBF, F5,30 = 9.32, n = 6 in each group, P < 0.0001; for LBF, F5,30 = 8.18, n = 6 in each group, P < 0.0001). These responses were saturated at approximately 20 V on both sides (Fig. 3A). The LN stimulation at 20 V showed a significant effect on the increases in both the MBF and LBF on the left side were above 10 and 2 Hz, respectively, (for MBF, F5,24 = 99.01, n = 5 in each group, P < 0.0001; for LBF, F5,24 = 76.82, n = 5 in each group, P < 0.0001). On the right side, LN stimulation also had a significant effect on the increases in both the MBF and LBF above 10 Hz (for MBF, F5,24 = 12.13, n = 5 in each group, P < 0.0001; for LBF, F5,24 = 18.33, n = 5 in each group, P < 0.0001). These responses were saturated at approximately 20 Hz on both sides (Fig. 3B). The mean changes in SABP following LN stimulation were too slight to account for the blood flow changes measured by the present methods (9.4 ± 2.7 mmHg, n = 5, for LN stimulation for 20 s with the supramaximal intensity (20 V) at 20 Hz using 2 ms pulses) (data not shown). MBF and LBF, and SABP changes were not evoked by the LN stimulation after the sectioning the more central portion of the LN than the LN stimulation site (data not shown). This indicated that the MBF increase evoked by LN stimulation was not due to stimulus spread to the trigeminal afferent or the parasympathetic nerves, which might occur around the LN.
Figure 2. Effects of electrical stimulation of the central cut end of LN on the blood flow in the masseter muscle (MBF) and the lower lip (LBF), and on the systemic arterial blood pressure (SABP).
The left LN was stimulated (•) for 20 s with various intensities (1–30 V) at 20 Hz using 2 ms pulses. Intensities (1–30 V) of the LN stimulation are shown below the stimulus marker. a.u., arbitrary units.
Figure 3. Stimulus intensity– and frequency–response relationships for the changes in the MBF and LBF evoked by LN stimulation.
Stimulation was at various intensities (1–30 V) and various frequencies (1–50 Hz). The intensity– (A) and frequency– (B) response curves of the left (ipsilateral, filled symbols) and right (contralateral, open symbols) sides in the masseter muscle (• and ○, n = 6 in each group) and the lower lip (▪ and □, n = 6 in each group) were generated using stimulus trains for the left LN at 20 Hz and at 20 V, respectively. Maximal increases in both the MBF and LBF in response to LN stimulation were taken as 100%. Each value is given as the mean ± s.e.m. The statistical significance of the differences was assessed by ANOVA followed by a post hoc test (Fisher's PLSD). *P < 0.05, **P < 0.01, ***P < 0.001 versus basal value (at intensity of 1 V and at frequency of 1 Hz). ††P < 0.01, †††P < 0.001, significant differences between the increases in blood flow recorded by LN stimulation on the left side and right side (ANOVA followed by a contrast test).
Effects of the pharmacological agents on the increases in the MBF, LBF and SABP evoked by LN stimulation
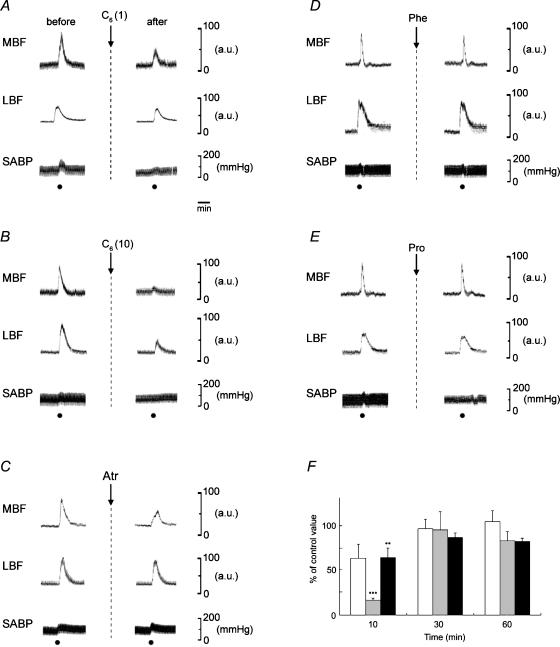
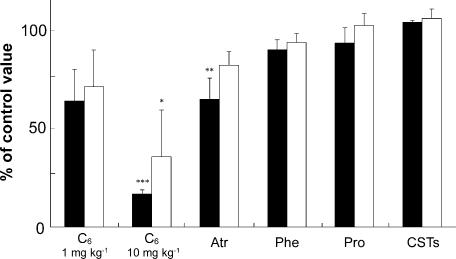
Figure 4 shows the effects of i.v. administration of C6 (A and B), atropine (C), phentolamine (D) and propranolol (E) on the increases in the left MBF and LBF, and SABP evoked by left LN stimulation for 20 s with the supramaximal intensity (20 V) at 20 Hz using 2 ms pulses. The mean changes in MBF and LBF evoked by the LN stimulation 10 min after treatment with C6 (1 and 10 mg kg−1), expressed as percentages of the control response, were 63.7 ± 15.8 and 16.7 ± 2.1%, and 71 ± 18.5 and 35 ± 23.8, respectively. There was a significant difference in the inhibitory effect of C6 at 10 mg kg−1 in both the MBF and LBF (for MBF, F3,16 = 10.71, n = 5 in each group, P < 0.0004; for LBF, F2,9 = 5.41, n = 4 in each group, P < 0.05). The atropine pretreatment (100 μg kg−1) reduced the increases in the MBF significantly (for MBF, F3,32 = 3.76, n = 9 in each group, P < 0.05), but not in the LBF (for LBF, F6,35 = 1.047, n = 11 in each group, NS) (Fig. 6). These responses in the MBF returned to almost the control level 60 min after the administration of each drug (Fig. 4F). Pretreatment with phentolamine or propranolol at a dose of 100 μg kg−1 had no effect on either response (for phentolamine, F2,12 = 1.82, n = 5 in each group, NS; for propranolol, F2,12 = 0.69, n = 5 in each group, NS) (Fig. 4D and E, and Fig. 6). The resting mean SABP level before pretreatment was 97.3 ± 6.1 mmHg, and 10 min after administration of C6 (1 and 10 mg kg−1); 87.8 ± 20.3 and 74 ± 16.4, atropine; 96.5 ± 14.6, phentolamine; 83.5 ± 5.1, or propranolol; 94.9 ± 8.4 mmHg, respectively. There were no significant differences in the SABP levels before and after the administration of each drug (F6,20 = 0.72, n = 4 in each group, NS).
Figure 4. Effects of pharmacological agents on the increases in the MBF and LBF on the left (ipsilateral) side evoked by left LN stimulation, and on the SABP.
Electrical stimulation was delivered (•) for 20 s with a supramaximal voltage of 20 V at 20 Hz using 2 ms pulses, before (control) and after (at 10 min) intravenous administration of hexamethonium (C6) at 1 and 10 mg kg−1 (A and B), atropine at 100 μg kg−1 (Atr; C), phentolamine at 100 μg kg−1 (Phe; D), and propranolol at 100 μg kg−1 (Pro; E). F, the MBF increases evoked by LN stimulation 10, 30 and 60 min after administration of C6 at 1 (white bars) and 10 (grey bars) mg kg−1, and Atr (black bars), are expressed as percentages of the pretreatment response and given as means ±s.e.m. The statistical significance of the differences in this pretreatment response (control) was assessed by ANOVA followed by a post hoc test (Fisher's PLSD) (**P < 0.01, ***P < 0.001).
Figure 6. Mean data for the effects of pharmacological agents and the sectioning the CST on the increases in the MBF and LBF evoked by LN stimulation.
Each value (mean ± s.e.m.) for the effects of C6 at 1 and 10 mg kg−1 (for MBF, n = 5; for LBF, n = 4), Atr at 100 μg kg−1 (for MBF, n = 9; for LBF, n = 11), Phe at 100 μg kg−1 (n = 5 in each group), Pro at 100 μg kg−1 (n = 5 in each group), and sectioning the CST (CSTs) (n = 5 in each group) on the increases in the MBF (white bars) and LBF (black bars) on the left side (ipsilateral) evoked by the left LN stimulation are expressed as percentages of the pretreatment response. The stimulation was delivered for 20 s with a supramaximal voltage (20 V) at 20 Hz using 2 ms pulses, and was carried out before (control) and 10 min after intravenous administration of each drug, or 10 min after sectioning the CST. The statistical significance of the differences in this pretreatment response (control) was assessed by ANOVA followed by a post hoc test (Fisher's PLSD) (*P < 0.05, **P < 0.01, ***P < 0.001).
Effects of phentolamine on vasoconstrictor responses induced by electrical stimulation of the peripheral cut end of the CST
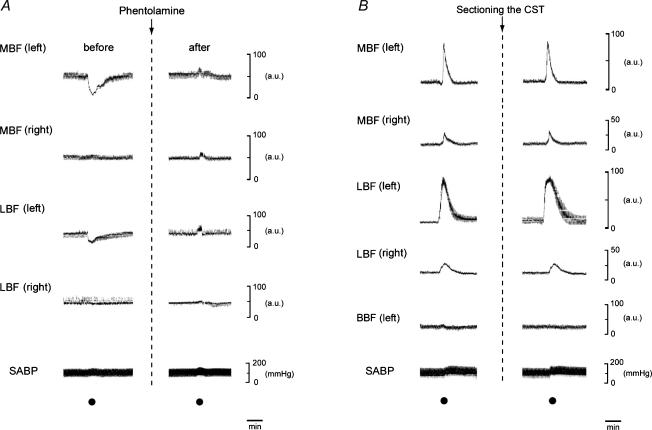
Figure 5A shows the effect of phentolamine on the blood flow changes in MBF, LBF and SABP induced by electrical stimulation of the peripheral cut end of the left CST. The CST stimulation reduced the blood flow level in both MBF and LBF in the left side, but not in the right side. These effects were eliminated by intravenous infusion of phentolamine (100 μg kg−1). Slight increases in SABP were elicited by CST stimulation regardless of the presence or absence of phentolamine.
Figure 5. Sympathetic effects on the blood flow parameters.
A, examples of the effects of the Phe (100 μg kg−1) on the blood flow changes in MBF, LBF and SABP induced by electrical stimulation of the peripheral cut end of the left superior CST. B, the effects of the sectioning left CST on the changes in MBF, LBF and biceps femoris muscle blood flow (BBF) and SABP evoked by electrical stimulation to the central cut end of the left LN. The CST and LN were stimulated (•) for 20 s with a supramaximal voltage (10 V) at 10 Hz; and for 20 s with a supramaximal voltage (20 V) at 20 Hz using 2 ms pulses, respectively. Electrical stimulations were carried out before (control) and after (at 10 min) intravenous administration of Phe, or 10 min after the sectioning the CST.
Effect of the sectioning the CST on the changes in the MBF, LBF, BBF and SABP evoked by LN stimulation
Figure 5B shows the effects of the section of left CST on the changes in MBF, LBF, BBF and SABP evoked by left LN stimulation. The sectioning of the CST did not alter the increases of either the MBF or LBF (F2,12 = 1.01, n = 5 in each group, NS) (Figs 5B and 6). The SABP levels were not different before and after the sectioning the CST (F6,21 = 0.60, n = 4 in each group, NS). The LN stimulation had no effects on the BBF for either intact or sectioned CST.
Effects of the C6 and atropine on the MBF increases evoked by TG stimulation
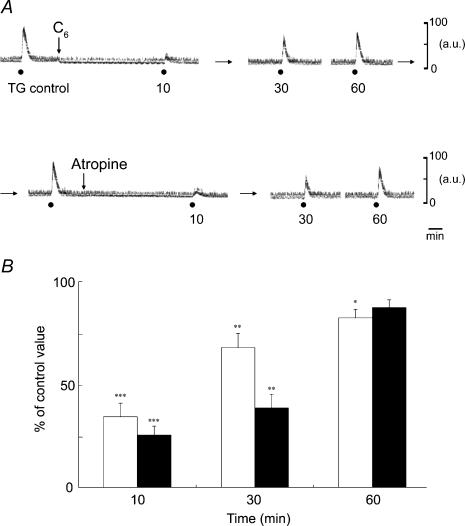
Electrical stimulation at sites within the left TG evoked MBF increases on the left side for 20 s with the 20 V at 20 Hz using 2 ms pulses. Figure 7A shows the effects of the C6 (10 mg kg−1, i.v.) and atropine (100 μg kg−1, i.v.) on the MBF increases elicited by TG stimulation. The mean data of changes in the MBF evoked by the TG stimulation 10, 30 and 60 min after treatment with C6 and atropine, expressed as percentages of the control response, were 34.5 ± 6.6 and 25.6 ± 4.4, 68.1 ± 6.9 and 38.7 ± 6.6, and 82.6 ± 4.1 and 87.6 ± 3.7%, respectively. There were significant differences in the inhibitory effects of both C6 and atropine (for C6, F3,12 = 28.6, n = 4 in each group, P < 0.0001; for atropine, F3,8 = 6.95, n = 3 in each group, P < 0.05). These responses recovered in a time-dependent manner, but remained significantly different from the initial control value 60 min after C6 administration (Fig. 7B). There were no significant differences between the SABP levels before and after the administration of each drug (for C6, F1,6 = 0.6, n = 4 in each group, NS; for atropine, F1,6 = 0.21, n = 4 in each group, NS) (data not shown).
Figure 7. Effects of pharmacological agents on the increases in MBF on the left (ipsilateral) side evoked by electrical stimulation of the left TG.
A, typical examples of the effects of the intravenous administration of C6 (10 mg kg−1) and Atr (100 μg kg−1) on the MBF increases evoked by TG stimulation (•) for 20 s with 20 V at 20 Hz using 2 ms pulses. B, the MBF increases evoked by TG stimulation 10, 30 and 60 min after administration of C6 (white bars, n = 4 in each group) and Atr (black bars, n = 3 in each group) are expressed as a percentage of the pretreatment response and given as means ± s.e.m. The statistical significance of the differences in this pretreatment response (control) was assessed by ANOVA followed by a post hoc test (Fisher's PLSD) (*P < 0.05, **P < 0.01, ***P < 0.001).
Effects produced by microinjection of lidocaine into the Vsp or SN on the MBF increase elicited by LN stimulation
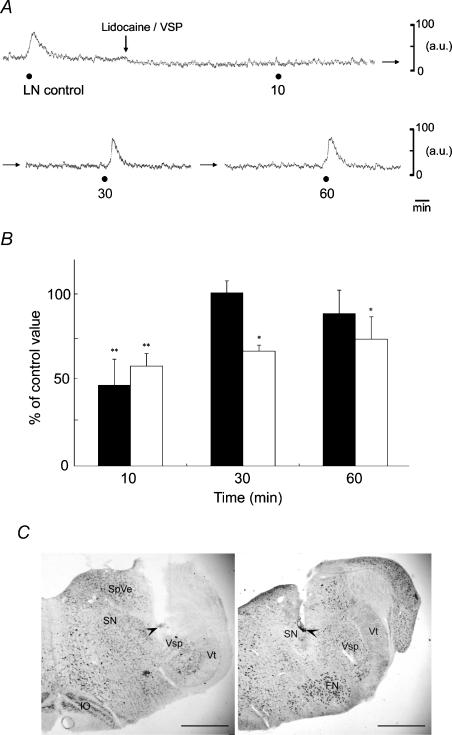
Local anaesthesia of the ipsilateral Vsp or SN by microinjection of lidocaine was produced to examine the contribution made by these areas to the MBF increases elicited by LN stimulation. Figure 8A shows the effects produced by microinjection of lidocaine (4%) in a volume of 0.3 μl per site into the Vsp on the MBF increases elicited by LN stimulation. The mean data of changes in the MBF elicited by LN stimulation 10, 30 and 60 min after microinjection of lidocaine into the Vsp and SN, expressed as percentages of the control response, were 46.9 ± 15.1 and 58 ± 7.4, 100.6 ± 6.9 and 66.8 ± 3.5, and 88.6 ± 13.7 and 73.8 ± 13%, respectively. There were significant differences in the inhibitory effects of lidocaine (for Vsp, F3,20 = 5.52, n = 6 in each group, P < 0.01; for SN, F3,12 = 5.53, n = 4 in each group, P < 0.05). These responses recovered in a time-dependent manner, but remained significantly different from the initial control value 60 min after the lidocaine microinjection into the SN (Fig. 8B). Typical microinjection sites are shown in Fig. 8C.
Figure 8. Effects produced by microinjection of lidocaine into the left (ipsilateral) trigeminal spinal nucleus (Vsp) and salivatory nuclei (SN) on the MBF increase elicited by left LN stimulation.
A, typical examples of the effects produced by microinjection of lidocaine (lignocaine; 4%) in a volume of 0.3 μl per site into the Vsp on the MBF increase elicited by LN stimulation (•), for 20 s with 20 V at 20 Hz using 2 ms pulses. B, the MBF increases evoked by LN stimulation 10, 30 and 60 min after microinjection of lidocaine into the Vsp (black bars, n = 6 in each group) and SN (white bars, n = 4 in each group) are expressed as percentages of the pretreatment response and given as means ± s.e.m. The statistical significance of the differences in this pretreatment response (control) was assessed by ANOVA followed by a post hoc test (Fisher's PLSD) (*P < 0.05, **P < 0.01, ***P < 0.001). C, photomicrographs of representative coronal sections stained with thionin through the medulla oblongata of the rat showing sites (arrowheads) at which microinjections of lidocaine were delivered to the Vsp (left panel) and SN (right panel). Scale bars represent 1 mm. FN, facial nucleus; IO, inferior olivary nucleus; SpVe, spinal vestibular nucleus; Vt, spinal trigeminal tract.
The establishment of the peripheral course underlying the MBF increase evoked by LN stimulation with a retrograde HRP-labelling method
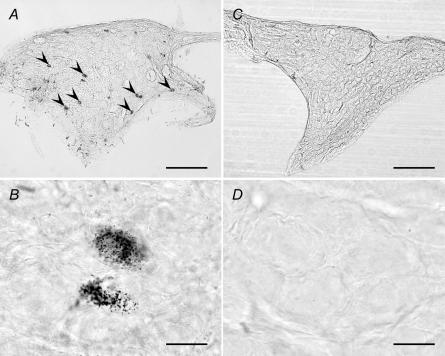
When WGA–HRP was injected into the masseter muscle, labelled neurones were ipsilaterally identified in the OG (Fig. 9A and B), but not PPG in both rostral and caudal areas (Fig. 9C and D). HRP-labelled neurones were also observed in the SCG, TG, trigeminal motor and mesencephalic trigeminal nuclei. These neurones contained reticular granules (a characteristic of HRP labels) in their cell bodies in a high-power photomicrograph (Fig. 9B). No HRP-labelled cells were identified in facial nuclei in any rat examined (data not shown). This indicated that there was no leakage of the WGA–HRP solution from the masseter muscle to surrounding tissues such as facial muscles.
Figure 9. WGA–HRP-labelled neurones in the autonomic ganglions.
Photomicrographs showing wheat germ agglutinin–horseradish peroxidase (WGA–HRP)-labelled neurones after an injection of WGA–HRP into the masseter muscle in the rat. High- and low-power photomicrographs of the OG (A and B) and the pterygopalatine ganglion (PPG) (C and D) are shown. Arrowheads indicate the WGA–HRP-labelled cells. The left side of each photomicrograph is the rostral. Bars, 200 μm (A and C) and 20 μm (B and D).
Discussion
The LN stimulation consistently increased the MBF in intensity- and frequency-dependent manners in all the animals examined in the present study (Figs 2 and 3). At least three possible mechanisms should be considered, as the blood flow increases could have been due to (i) an effect secondary to the SABP increase, (ii) axon reflex vasodilatation mediated via activation of sensory fibres with collateral branches to the blood vessels of the tongue, or (iii) an autonomic reflex mechanism (parasympathetic or sympathetic reflex vasodilatation). However, the subsequent experiments reported here allowed the exclusion of (i) and (ii), and supported (iii), the last of these possible mechanisms, as will be described below.
The observed MBF increases appeared not to be secondary to changes in the SABP because (1) the blood flow increase occurring on the ipsilateral side was significantly larger than that on the contralateral side (Figs 2 and 3), and (2) there was no significant increase in the SABP at the LN stimulation. These results suggested that the blood flow increase elicited by the LN stimulation was not a passive result of any evoked blood pressure change, and that it is justifiable to refer to it as ‘vasodilatation’.
The autonomic ganglion cholinergic blocker C6 (10 mg kg−1) significantly reduced the MBF increase evoked by LN stimulation (Fig. 6), indicating that the efferent pathway of the autonomic nervous system rather than axon reflexes was responsible for this response. Pretreatment with either phentolamine (α-adrenoceptor antagonist, 100 μg kg−1) or propranolol (β-adrenoceptor antagonist, 100 μg kg−1) failed to affect the response (Fig. 4D and E, and Fig. 6), and the sectioning the CST did not affect the response either (Figs 5B and 6). These observations effectively ruled out the involvement of sympathetic fibres as the efferent mediator of the present response. Electrical stimulation of the CST consistently reduced the MBF, and it was completely eliminated by administration of phentolamine (Fig. 5A), in accord with the observation of Granstam & Nilsson (1990). The MBF increase could be also elicited by TG stimulation in the present study. Pretreatment with C6 (10 mg kg−1) markedly reduced the response, indicating that the MBF increase induced by TG stimulation in the rat was mediated via activation of the parasympathetic mechanism, but not the antidromic mechanism, through the trigeminal nerve. This is in accord with the observation of Date et al. (2000) that the TG stimulation-induced LBF increase in cats is entirely mediated through the parasympathetic reflex mechanism. Further, this was supported by the findings that the MBF increase evoked by electrical stimulation of the LN was significantly attenuated by microinjection of lidocaine into the Vsp or SN (Fig. 8). The blood flow increases obtained by the present LDF method seem likely to be due to the MBF, but unlikely to increase in the adjacent tissues, such as parotid gland, because (1) the blood flow measurement by use of LDF is limited to within 1–1.5 mm in diameter when LDF probes are 1 mm in diameter, and (2) the site of LDF measurement on the masseter muscle was 10–15 mm apart from parotid gland in rats. On the basis of the data presented here, we suggest that the LN must be regarded as the sensory link involved in evoking parasympathetic reflex vasodilatation in the rat masseter muscle.
The pharmacological analysis also showed that the parasympathetic vasodilator response in the masseter muscle was partly sensitive to the antimuscarinic agent atropine (100 μg kg−1) (Figs 4, 6 and 7), suggesting that this parasympathetic reflex vasodilatation was mediated by final neurones via cholinergic and noncholinergic responses. In contrast, Faraci et al. (1986) reported that the MBF increase is insensitive to atropine, and that in anaesthetized and bilateral cervical sympathetic denervated rats, seizures produced by bicuculline or pentylenetetrazole markedly induce an MBF increase that is not blocked by atropine. The precise reasons for the differences in the evaluations of atropine sensitivity are unclear, but may be due to differences in blood flow measurements or in the activation method of parasympathetic fibres. Furthermore, atropine-resistant parasympathetic vasodilatation in the lower lip was evoked by the LN stimulation in all the rats examined (Figs 4C and 6). These atropine-resistant responses have been suggested to be mediated by vasoactive intestinal peptide (VIP), as reported with previous reports on the cat and rat (Izumi & Karita, 1990, 1992, 1993; Izumi & Ito, 1998; Mizuta et al. 2000; Mizuta & Izumi, 2004). Considering these differences in atropine sensitivity between MBF and LBF, the neural regulatory mechanism in the MBF apparently differs from that in LBF with respect to efferent fibres. When WGA–HRP was injected into the masseter muscle, labelled neurones were identified in the OG, but not the PPG (Fig. 9). This indicates that the rat masseter muscle is innervated by parasympathetic vasodilator fibres originating from cell bodies in the OG. Anatomical evidence shows that the parasympathetic nerves of the cephalic circulation are most abundant in the arteries supplying the masseter and lingual muscles in the cat (Gibbins et al. 1984) and rabbit (Zhu et al. 1997), and that these are composed of fibres containing VIP and/or NADPH diaphorase (demonstrating nitric oxide synthase). These nerves presumably employ acetylcholine, as the coexistence of immunoreactivity to VIP and choline acetyltransferase has been explicitly demonstrated in the cranial parasympathetic ganglia of rats (Leblanc et al. 1987).
We have not been able to identify other studies with evidence that parasympathetic cholinergic and noncholinergic vasodilator fibres exist in the rat masseter muscle, and the present study may be considered to be the first published evidence of this. Masseter muscle fatigue and soreness are usually the most common findings in the functional disorders of the masticatory system (Yemm, 1971). The underlying mechanisms of masseter muscle pain and dysfunction are not fully understood. The ischaemia in the masseter muscle may play a role in the development of craniomandibular disorders, because even low levels of sustained activity can give rise to blood flow impairment (Rasmussen et al. 1977). Under such conditions, an increase in blood flow to the masseter muscle, which occurs via the trigeminal mediated reflex, may be important in the maintenance of blood flow in the masseter muscle.
It is hoped that further studies on the properties of the afferent input, the pathways followed by the efferent nerve fibres, and the central mechanisms involved in this reflex will provide data enabling a better understanding of the physiological role of somatoparasympathetic reflex vasodilatation in jaw muscles.
Acknowledgments
This study was partly supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 15591962; H. Izumi), the Akiyama Foundation (2004; H. Izumi), High-Tech Research Center Project for Private Universities: matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology: 2003; H. Izumi), and the Northern Advancement Center for Science and Technology of Japan (2004; H. Ishii).
References
- Alm A. The effect of stimulation of the cervical sympathetic chain on regional cerebral blood flow in monkeys. A study with radioactively labelled microspheres. Acta Physiol Scand. 1975;93:483–489. doi: 10.1111/j.1748-1716.1975.tb05839.x. [DOI] [PubMed] [Google Scholar]
- Bolme P, Novotny J, Uvnas B, Wright PG. Species distribution of sympathetic cholinergic vasodilator nerves in skeletal muscle. Acta Physiol Scand. 1970;78:60–64. doi: 10.1111/j.1748-1716.1970.tb04639.x. [DOI] [PubMed] [Google Scholar]
- Date H, Kato M, Izumi H. Involvement of two different mechanisms in trigeminal ganglion-evoked vasodilatation in the cat lower lip: role of experimental conditions. J Auton Nerv Syst. 2000;79:84–92. doi: 10.1016/s0165-1838(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Delcanho RE, Kim YJ, Clark GT. Haemodynamic changes induced by submaximal isometric contraction in painful and non-painful human masseter using near-infra-red spectroscopy. Arch Oral Biol. 1996;41:585–596. doi: 10.1016/0003-9969(96)00009-x. [DOI] [PubMed] [Google Scholar]
- Edwall B, Gazelius B, Berg JO, Edwall L, Hellander K, Olgart L. Blood flow changes in the dental pulp of the cat and rat measured simultaneously by laser Doppler flowmetry and local 125I clearance. Acta Physiol Scand. 1987;131:81–91. doi: 10.1111/j.1748-1716.1987.tb08208.x. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Motley MG, Heistad DD. Activation of a cranial vasodilator pathway during seizures in rats. Am J Physiol Heart Circ Physiol. 1986;251:H969–975. doi: 10.1152/ajpheart.1986.251.5.H969. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Brayden JE, Bevan JA. Perivascular nerves with immunoreactivity to vasoactive intestinal polypeptide in cephalic arteries of the cat: distribution, possible origins and functional implications. Neuroscience. 1984;13:1327–1346. doi: 10.1016/0306-4522(84)90301-4. [DOI] [PubMed] [Google Scholar]
- Granstam E, Nilsson SF. Non-adrenergic sympathetic vasoconstriction in the eye and some other facial tissues in the rabbit. Eur J Pharmacol. 1990;175:175–186. doi: 10.1016/0014-2999(90)90228-x. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia: W. B. Saunders; 1996. [Google Scholar]
- Hidaka O, Yanagi M, Takada K. Mental stress-induced physiological changes in the human masseter muscle. J Dent Res. 2004;83:227–231. doi: 10.1177/154405910408300308. [DOI] [PubMed] [Google Scholar]
- Izumi H, Ito Y. Sympathetic attenuation of parasympathetic vasodilatation in oro-facial areas in the cat. J Physiol. 1998;510:915–921. doi: 10.1111/j.1469-7793.1998.915bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Karita K. The effects of capsaicin applied topically to inferior alveolar nerve on antidromic vasodilatation in cat gingiva. Neurosci Lett. 1990;112:65–69. doi: 10.1016/0304-3940(90)90323-2. [DOI] [PubMed] [Google Scholar]
- Izumi H, Karita K. Somatosensory stimulation causes autonomic vasodilatation in cat lip. J Physiol. 1992;450:191–202. doi: 10.1113/jphysiol.1992.sp019123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Karita K. Innervation of the cat lip by two groups of parasympathetic vasodilator fibres. J Physiol. 1993;465:501–512. doi: 10.1113/jphysiol.1993.sp019690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Halliwill JR. Sympathetic vasodilatation in human limbs. J Physiol. 2000;526:471–480. [PubMed] [Google Scholar]
- Kaji A, Maeda T, Watanabe S. Parasympathetic innervation of cutaneous blood vessels examined by retrograde tracing in the rat lower lip. J Auton Nerv Syst. 1991;32:153–158. doi: 10.1016/0165-1838(91)90065-b. [DOI] [PubMed] [Google Scholar]
- Kim S, Liu M, Markowitz K, Bilotto G, Dorscher-Kim J. Comparison of pulpal blood flow in dog canine teeth determined by the laser Doppler and the 133xenon washout methods. Arch Oral Biol. 1990;35:411–413. doi: 10.1016/0003-9969(90)90189-h. [DOI] [PubMed] [Google Scholar]
- Koeda S, Yasuda M, Izumi H. Species differences in the reflex effects of lingual afferent nerve stimulation on lip blood flow and arterial pressure. J Comp Physiol [B] 2003;173:629–636. doi: 10.1007/s00360-003-0363-7. [DOI] [PubMed] [Google Scholar]
- Kuchiiwa S, Kuchiiwa T. Autonomic and sensory innervation of cat molar gland and blood vessels in the lower lip, gingiva and cheek. J Auton Nerv Syst. 1996;61:227–234. doi: 10.1016/s0165-1838(96)00087-2. [DOI] [PubMed] [Google Scholar]
- Leblanc GG, Trimmer BA, Landis SC. Neuropeptide Y-like immunoreactivity in rat cranial parasympathetic neurons: coexistence with vasoactive intestinal peptide and choline acetyltransferase. Proc Natl Acad Sci U S A. 1987;84:3511–3515. doi: 10.1073/pnas.84.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund N, Bengtsson A, Thorborg P. Muscle tissue oxygen pressure in primary fibromyalgia. Scand J Rheumatol. 1986;15:165–173. doi: 10.3109/03009748609102084. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Ikehara A, Nokubi T, Morimoto T. Inhibitory effect of sympathetic stimulation on activities of masseter muscle spindles and the jaw jerk reflex in rats. J Physiol. 1995;483:239–250. doi: 10.1113/jphysiol.1995.sp020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Izumi H. Bulbar pathway for contralateral lingual nerve-evoked reflex vasodilatation in cat palate. Brain Res. 2004;1020:86–94. doi: 10.1016/j.brainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Karita K, Izumi H. Parasympathetic reflex vasodilatation in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2000;279:R677–683. doi: 10.1152/ajpregu.2000.279.2.R677. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. San Diego, California: Academic Press; 1998. [Google Scholar]
- Rasmussen OC, Bonde-Petersen F, Christensen LV, Moller E. Blood flow in human mandibular elevators at rest and during controlled biting. Arch Oral Biol. 1977;22:539–543. doi: 10.1016/0003-9969(77)90052-8. [DOI] [PubMed] [Google Scholar]
- Stern MD, Lappe DL, Bowen PD, Chimosky JE, Holloway GA, Keiser HR, Bowman RL. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol Heart Circ Physiol. 1977;232:H441–448. doi: 10.1152/ajpheart.1977.232.4.H441. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- Yemm R. Comparison of the activity of left and right masseter muscles of normal individuals and patients with mandibular dysfunction during experimental stress. J Dent Res. 1971;50:1320–1323. doi: 10.1177/00220345710500053901. [DOI] [PubMed] [Google Scholar]
- Zhu BS, Blessing WW, Gibbins IL. Parasympathetic innervation of cephalic arteries in rabbits: comparison with sympathetic and sensory innervation. J Comp Neurol. 1997;389:484–495. doi: 10.1002/(sici)1096-9861(19971222)389:3<484::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]