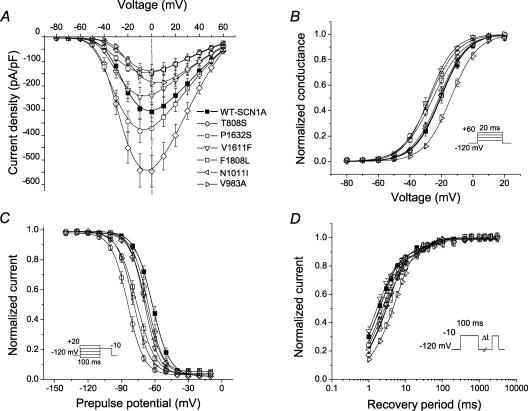
Figure 3. Inactivation and activation properties of ICEGTC-associated mutants.
A, current–voltage relationships of whole-cell currents from transiently transfected tsA201 cells. Currents were elicited by test pulses to various potentials (B, inset) and normalized to cell capacitance (WT-SCN1A, n = 14; T808S, n = 12; P1632S, n = 10; V1611F, n = 12; F1808L, n = 11; N1011I, n = 6; V983A, n = 9). T808S current density is significantly larger than WT between −50 and +60 mV (P < 0.05). P1632S current density is significantly larger than WT between −40 and −20 mV (P < 0.05). F1808L and N1011I current density is significantly smaller than WT between −20 and +60 mV (P < 0.05). V983A current density is significantly smaller than WT between −30 and 0 mV (P < 0.05). B, voltage dependence of activation. The voltage dependence of channel activation was estimated by measuring peak sodium current during a variable test potential step from a holding potential of −120 mV. The current at each membrane potential was divided by the electrochemical driving force for sodium ions and normalized to the maximum sodium conductance. C, voltage dependence of inactivation. The two-pulse protocol outlined in the inset was used to examine channel availability after conditioning at various potentials. Currents were normalized to the peak current amplitude. D, recovery from fast inactivation. Channels were inactivated by a 100 ms pulse and then stepped to −120 mV for increasingly long periods. Currents were normalized to the peak current amplitude measured during the inactivation pulse and fitted to a two-exponential function generating fast and slow recovery time constants. Fitted values from these experiments are provided in Table 1.