Fig. 1.
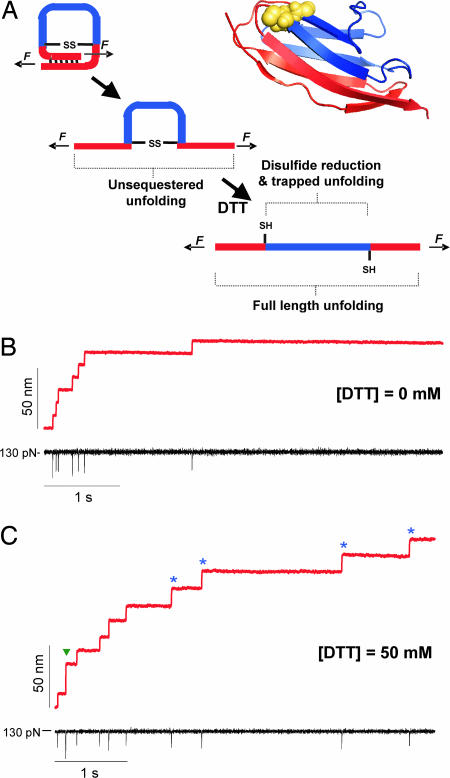
Force–clamp spectroscopy identifies single thiol/disulfide exchange events under a stretching force. (A) An engineered disulfide bond was introduced between the 32nd and the 75th residue of the 27th Ig-like domain of cardiac titin (I27G32C–A75C). In the ribbon diagram of I27G32C–A75C, mutated residues 32 and 75 are yellow spheres, residues 1–31 and 76–89 are pictured in red (unsequestered residues), and 33–74, behind the disulfide bond, are in blue (trapped residues). The cartoons on the left depict the three sequential events that take place when we apply a mechanical force to the I27G32C–A75C protein. Applying a mechanical force first triggers the unfolding and extension of the protein, up to the position of the disulfide bond. We call this initial elongation unsequestered unfolding. If DTT is present in the bathing solution, disulfide bond reduction can occur, allowing for the extension of the trapped residues. (B) Force–clamp experiment showing the stepwise elongation (red trace) of an (I27G32C–A75C)8 polyprotein pulled at a constant force of 130 pN (black trace) in the absence of DTT. Seven steps of equal size, ≈10.6 nm, mark the sequential unfolding of the unsequestered region of seven I27G32C–A75C modules in the polyprotein. The brief downward deflections in the force (black trace) are due to lag in the feedback electronics after each unfolding event. (C) When the same experiment is repeated in the presence of 50 mM DTT, we again observe a series of ≈10.8 nm steps corresponding to the unsequestered unfolding events, followed by several ≈13.8 nm steps (blue stars), which mark thiol/disulfide exchange events and the subsequent extension of the trapped residues. In this trace, we also observe an event with an amplitude of ≈24.0 nm (green arrow), which corresponds to the unfolding of a single fully reduced I27 module.