Fig. 3.
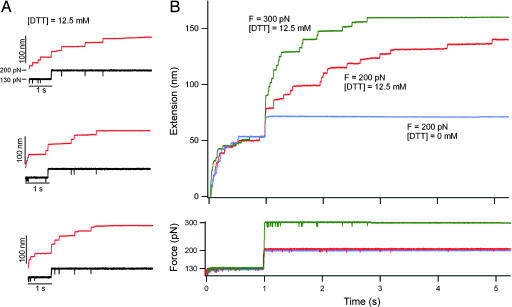
Ensemble measurements of the kinetics of thiol/disulfide exchange. (A) Three recordings are shown of single (I27G32C–A75C)8 polyproteins that were extended with the same double-pulse protocol shown in Fig. 2B: 12.5 mM DTT, F = 130 pN for 1 s, and then F = 200 pN. The stochastic nature of both the unsequestered unfolding events as well as of the thiol/disulfide exchange events becomes apparent when comparing these recordings. (B Upper) A four-trace average (red trace) of the double-pulse experiments shown in A and Fig. 2B demonstrates the methods used to build up an ensemble of recordings under a set of conditions. Similar four-trace averages are shown for data obtained under two other conditions: 12.5 mM DTT, F = 130 pN for 1 s then F = 300 pN (green trace); and 0 mM DTT, F = 130 pN for 1 s then F = 200 pN (blue trace). Notice that the time course of unsequestered unfolding during the first pulse is similar under all conditions. (B Lower) The averaged force traces are shown.