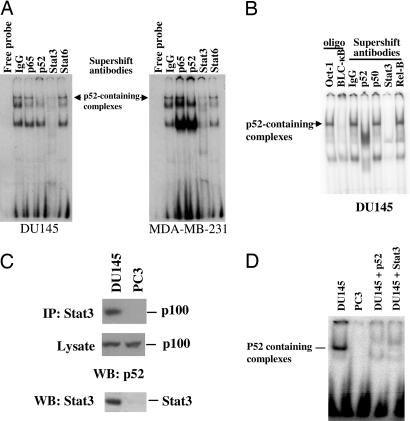
Fig. 1.
NF-κB p52 DNA binding activity in DU145 and MDA-MB-231 cells that contains Stat3 protein in p52-containing complexes. (A) NF-κB p52 EMSAs were carried out with nuclear fractions generated from DU145 and MDA-MB-231 cells by using a probe (BLC-κB, 5′-GGGAGATTTG-3′) specifically bound by RelB:p52 dimers. Specificity of p52 DNA binding was confirmed by supershift with antibodies specifically against p52 or control antibodies (IgG). A complex formed by nuclear extracts from both DU145 and MDA-MB-231 cells was supershifted with antibodies against Stat3 but not with antibodies against either NF-κB p65 or Stat6. (B) NF-κB p52 EMSA was carried out with nuclear fractions generated from DU145 cells by using the BLC-κB probe. Specificity of p52 DNA binding was confirmed by competition with excess unlabeled Oct-1 oligonucleotides (Oligo, Oct-1) or BLC-κB oligonucleotides (Oligo, BLC-κB) or supershift with antibodies against p52, p50, Stat3, RelB, or control antibodies (IgG). (C) Stat3 is associated with p100 protein. Cell extracts from DU145 and PC3 cells were subjected to immunoprecipitation (IP) with antibodies against Stat3. Precipitates or lysates were separated by SDS/PAGE and analyzed by immunoblotting (WB) with antibodies against p100. Expression of Stat3 was determined by immunoblotting (WB) the cell extracts with antibodies against Stat3. (D) Stat3 is associated with p52. Cell extracts from DU145 and PC3 cells were subjected to immunoprecipitation with antibodies against Stat3. The eluted immunoprecipitants were used for EMSAs using radiolabeled Blc-κB probe. Specificity of p52 DNA binding was confirmed by supershift with the antibodies specifically against p52 and Stat3 in DU145 cells.