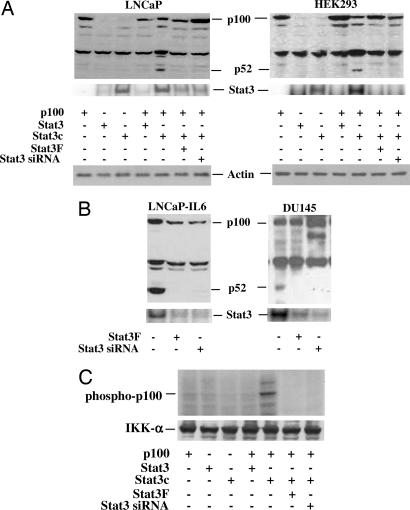
Fig. 2.
Stat3 induces p52 production. (A) Active Stat3 induces p52 production in LNCaP and HEK293 cells. Cells were cotransfected with plasmids containing p100, Stat3, constitutively active Stat3 mutant (Stat3c), dominant-negative Stat3 mutant (Stat3F), or Stat3 siRNA. All transfections contained 2.0 μg of total plasmid DNA. After 48 h cells were lysed in RIPA buffer, and Western blot analyses were performed by using antibodies against p52. (B) Inhibition of p52 production by blocking Stat3 activation in LNCaP-IL6 and DU145 cells. LNCaP-IL6 and DU145 cells were transfected with dominant-negative Stat3 mutant (Stat3F), Stat3 siRNA, or control plasmid. All transfections contained 2 μg of total plasmid DNA. Cells were lysed after 48 h, and Western blot analyses were performed by using antibodies against p52. Stat3 activity was analyzed by EMSAs by using radiolabeled probe containing consensus Stat3 DNA binding sequence (5′-GATCCTTCTGGGAATTCCTAGATC). (C) Kinase assay to show the phosphorylation of p100 by IKKα. Lysates from Stat3, Sta3c, Stat3F, and Stat3 siRNA-transfected LNCaP cells were immunoprecipitated with anti-IKKα antibodies, and the immunoprecipitated enzyme was used to phosphorylate p100 in vitro. Reactions were stopped with SDS loading buffer after 30 min, loaded for SDS/PAGE, and transferred to a nitrocellulose membrane. The phosphorylated p100 was visualized by autoradiography. The membrane was reprobed with anti-IKK-α antibodies to normalize for equal amounts of kinase in each reaction.