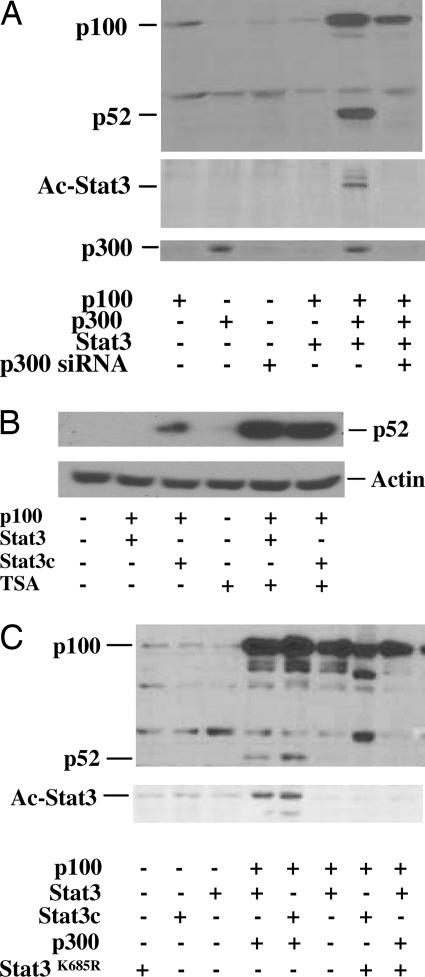
Fig. 3.
Stat3 acetylation by CBP/p300 induces processing of p100 to p52 in LNCaP cells. (A) Cells were cotransfected with plasmids containing p100, Stat3, p300, or p300 siRNA (5′-GAGGATATTTCAGAGTCTA-3′). All transfections contained 2 μg of total plasmid DNA. Cells were lysed after 48 h, and Western blot analyses were performed by using antibodies against p52. Stat3 acetylation (Ac-Stat3) was detected by immunoprecipitation of the lysates with anti-Stat3 and Western blot with antibodies against acetylated lysine. (B) TSA enhances Stat3-induced processing of p100 to p52. HEK293 cells were cotransfected with p100 and Stat3 or Stat3c, and the cells were treated with or without 0.2 μM TSA. The cell extracts were used for Western blot analysis by using antibody against p52. (C) Stat3 defective for acetylation at lysine (Stat3K685R) lost the ability to induce processing of p100 to p52. LNCaP cells were cotransfected with plasmids containing p100, Stat3, Stat3c, p300, or Stat3K685R. Cells were lysed after 48 h, and Western blot was performed by using antibodies against p52. Stat3 acetylation (Ac-Stat3) was detected by immunoprecipitation of the lysates with anti-Stat3 and Western blot with antibodies against acetylated lysine.