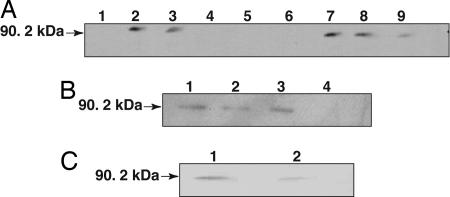
Fig. 2.
Immunoblot analysis of subcellular localization of Rpg1. (A) Crude Rpg1 protein extracted from 10-day-old Morex seedling leaves was fractionated by centrifugation and visualized by immunoblotting. Lane 1, cell wall debris retained by Mira cloth filter; lane 2, total extract after filtration; lane 3, 3,000 × g × 15 min supernatant; lane 4, 3,000 × g × 15 min pellet; lane 5, 15,000 × g × 15 min pellet; lane 6, empty; lane 7, 15,000 × g × 15 min supernatant; lane 8, 100,000 × g × 2 h supernatant; lane 9, 100,000 × g × 2 h pellet. Approximately 25 μg of total protein were loaded per lane. (B) The 100,000 × g pellet was treated with either 100 mM Na2CO3 (pH 11.5) or proteinase K (100 μg/ml) at 0°C for 30 min. The reaction mixtures were centrifuged at 14,000 × g, and the supernatant and precipitate were subjected to SDS/PAGE followed by Western blot analysis. Lanes 1 and 2, Na2CO3-treated microsomal pellet and supernatant; lanes 3 and 4, proteinase K-treated microsomal pellet and supernatant. (C) The 100,000 × g pellet was separated by two-phase aqueous partitioning (28), and the products were analyzed by SDS/PAGE and immunoblotting. Assays of marker enzymes for plasma membrane, endoplasmic reticulum, and mitochondria were as described in ref. 29. Lane 1, intracellular membranes; lane 2, plasma membrane.