Abstract
There is evidence that changes in perinatal nutrition programme the development of relative fat mass and the regulation of appetite in adult life. These studies have been primarily in the rodent utilizing maternal overnutrition or undernutrition imposed at different stages of pregnancy and beyond, mapping of neuropeptide localization and activity and appropriate null mutant models. Whilst the rodent offers significant advantages in terms of a short gestation and the availability of useful transgenic and null mutant models, there are also advantages to using an animal model more akin to the human, in which all components of the ‘fat–brain axis’ are present before birth, such as the sheep. This review summarizes recent work on the expression and localization of the ‘appetite regulatory’ peptides in the fetal rodent and sheep hypothalamus and their potential role in the early programming of postnatal appetite and obesity.
The early origins of obesity
During the past two decades there has been a marked increase in the global prevalence of adult and childhood obesity and currently more than 50% of all adults in the United States and the United Kingdom are overweight, i.e. have a body mass index (BMI) of greater than 25 kg m−2 (James, 1996; Campfield et al. 1998; Flegal et al. 2002; Ogden et al. 2002). An increase in the prevalence of obesity (BMI > 30 kg m−2) is associated with an increase in a range of co-morbidities including type 2 diabetes, high blood pressure and ischaemic heart disease (James, 1996) and in this context it is of interest that a range of epidemiological, clinical and experimental studies have shown that there is a relationship between the fetal nutritional environment and patterns of adult adiposity. A number of studies have reported that there is a J shaped or U shaped relationship between birth weight and adult fat mass, with a higher prevalence of adult obesity occurring in individuals who were of either low or high birth weight. There are associations between maternal and paternal birth weight with offspring birth weight and where adjustments for maternal BMI have been able to be made (Maffeis et al. 1994; Curhan et al. 1996a; Curhan et al. 1996b; Parsons et al. 2001), the relationship between birth weight and adult BMI has diminished. A recent study reported that there was a weak but positive relationship between birth weight and adult BMI and that this relationship was largely accounted for by maternal weight, i.e. heavier mothers had heavier babies and these babies went on to have a high BMI in adult life (Parsons et al. 2001). There is additional evidence, however, that the positive associations between birth weight and later BMI may represent an association of birth weight with lean, rather than adipose tissue. This indicates the importance of determining body composition, rather than solely BMI, in long-term follow-up studies (Singhal et al. 2003). In pregnancies complicated by maternal diabetes mellitus, gestational diabetes or even mildly impaired glucose tolerance, the offspring are at risk of developing obesity (Dorner & Plagemann, 1994; Buchanan & Kjos, 1999); and in another study of infants of diabetic mothers, 50% had weights greater than the 90th percentile at birth and at 8 years of age (Silverman et al. 1991).
Whilst people who were small babies tend to have a lower BMI in adult life, these individuals also tend to have a more abdominal distribution of adipose tissue, a significantly reduced muscle mass and a high overall body fat content in adolescent and adult life despite their lower BMI (Law et al. 1992; Fall et al. 1995; Malina et al. 1996; Okosun et al. 2000; Loos et al. 2001; Loos et al. 2002; Singhal et al. 2003). This is significant because central obesity is associated with the clustering of pathologies that defines the insulin resistance or metabolic syndrome (hypertension, dyslipidaemia, hyperinsulinism, impaired glucose tolerance or type 2 diabetes) (Reaven, 1988). Exposure to a reduced nutrient supply in early pregnancy, as occurred in the Dutch Winter Famine in 1944–45, also resulted in an increase in body weight, BMI and waist circumference at 50 years of age for the offspring (Ravelli et al. 1976). Interestingly, Parsons et al. (2001) found that men with a lower birth weight who then achieved a greater proportion of their adult height by 7 years of age had a risk of obesity comparable with that for men with higher birth weights. Based on this series of epidemiological studies, it has been suggested that the influence of maternal weight on the relationship between birth weight and subsequent BMI may operate through an impact of maternal and hence fetal nutrient supply. During the past decade many experimental studies have investigated the impact of varying maternal and hence fetal nutrition on patterns of postnatal growth, insulin secretion, the insulin sensitivity of the postnatal liver, skeletal muscle, adipose tissue and glucose tolerance and these important studies have been the focus of several recent reviews (Holemans et al. 1996; Hales et al. 1997; Hoet & Hanson, 1999; Hales & Ozanne, 2003; Armitage et al. 2004; McMillen et al. 2004). A series of studies have also highlighted, however, the possibility that varying maternal nutrition during critical windows of development may also alter the level of energy intake in the offspring through inducing changes in the expression, localization and action of specific neuropeptides in the appetite regulatory network present within the brain. This review will therefore focus on the nature and role of such changes in the central component of the energy regulating system in the early programming of adult obesity.
The appetite regulatory neural network
A range of appetite regulatory neuropeptides, including primarily the appetite stimulatory neuropeptides neuropeptide Y (NPY) and agouti-related protein (AgRP), and the appetite inhibitory neuropeptide precursor molecule pro-opiomelanocortin (POMC, precursor for α-melanocyte-stimulating hormone, αMSH) and the neuropeptide cocaine- and amphetamine-regulated transcript (CART), are expressed within the adult hypothalamus and act together to regulate energy balance (Friedman & Halaas, 1998; Schwartz, 2001). NPY is predominantly localized in the hypothalamic arcuate nuclei (ARC) and NPY neurones project to the paraventricular nucleus (PVN), dorsomedial nucleus (DMN), the perifornical region and the lateral hypothalamic area (LHA; Grove & Smith, 2003). NPY neurones are able to respond to a range of peripheral nutrient and hormonal metabolic signals such as glucose, insulin, and the adipocyte derived hormone, leptin. A long form variant of the leptin receptor is highly expressed on cell bodies in the ARC and DMN, and increases in circulating leptin concentrations during periods of increased food intake results in a decrease in hypothalamic NPY mRNA and a subsequent fall in energy intake (Schwartz, 2001). AgRP is coexpressed with NPY in the ARC and is an endogenous antagonist of the anorexigenic melanocortin receptors MC3-R and MC4-R in the PVN and other hypothalamic regions. The POMC derived peptide, αMSH, is an endogenous anorexigenic peptide which acts at the melanocortin receptors to suppress food intake, while leptin acts to up-regulate POMC expression within the ARC and thereby limits energy intake (Schwartz, 2001). The neuropeptide CART is colocalized within POMC neurones in the hypothalamus and also acts to suppress food intake.
Adult obesity is associated with relatively high circulating leptin concentrations, and the tendency to gain weight in some non-obese populations with high basal leptin concentrations may indicate an underlying role for leptin resistance in obesity (Chessler et al. 1998; Lindroos et al. 1998; Lissner et al. 1999). It has been proposed that elevated plasma levels of leptin result in an uncoupling of the action of leptin at its receptors in the hypothalamus, thereby disrupting signal transduction pathways which are required for the suppression of appetite by an increase in circulating leptin (Kieffer et al. 1996; Ahima & Flier, 2000). Alternatively, it has also been suggested that elevated plasma leptin is associated with impaired blood–brain leptin transport, and hence apparent central resistance to the leptin signal (Banks et al. 1999).
Development of the appetite regulatory system in the rodent
The development of the hypothalamic appetite regulatory network in rodents such as the rat or mouse occurs predominantly after birth. Whilst NPY is present within the fetal ARC from as early as 14.5 days gestation, NPY/AgRP projections between the ARC and DMN are not complete until some 10–11 days after birth and NPY containing projections to the PVN do not fully develop until around 15–16 days (Allen et al. 1984; Woodhams et al. 1985; Kagotani et al. 1989; Grove & Smith, 2003). During the first week after birth there appears to be a relative dominance of NPY and αMSH innervation of the PVN by efferents derived from the brainstem, rather than from the ARC, and it has therefore been suggested that vagal sensory information from the gut relating to gut fullness may be important in regulating feeding behaviour in the rat pup throughout this period (Grove & Smith, 2003). There is also transient expression of NPY in the DMN, the perifornical region and the LHA during the postnatal period and POMC, AgRP and MC4-R mRNAs are also all present within the rat hypothalamus through this postnatal period. In mice, projections from the ARC to other areas of the hypothalamus also develop during the postnatal period, with projections to the dorsomedial hypothalamus (DMH), PVN and LHA established in sequence between postnatal days 5 and 16 (Bouret et al. 2004a).
Early programming of appetite in the rodent
A series of early studies demonstrated that the amount of food consumed during suckling in the rat plays an important role in determining subsequent food intake in later life (Oscai & McGarr, 1978). When postnatal overnutrition is induced in rats by rearing in small litters of only three pups, they show an increased early weight gain and fat deposition, followed by hyperphagia, obesity, hyperleptinaemia, hyperglycaemia, hyperinsulinaemia and insulin resistance (Plagemann et al. 1992, 1999a, d). Leptin has a lower inhibitory effect on the appetite stimulatory neurones of the ARC in these animals as young adults whereas insulin and leptin tend to exert greater inhibitory actions in the ventromedial nucleus (VMN) (Davidowa & Plagemann 2000, 2001). Neurones in the VMN also have altered responses to NPY and there are altered responses to both orexigenic (AgRP) and anorexigenic (αMSH, CART) neuropeptides in the PVN in the young adult after postnatal overfeeding (Heidel et al. 1999; Davidowa et al. 2002, 2003; Li et al. 2002).
When mild hyperglycaemia is induced by streptozotocin-induced gestational diabetes from early pregnancy, pups are macrosomic at birth and maintain an accelerated growth during the first 10 weeks of age (Oh et al. 1988). In macrosomic, hyperinsulinaemic pups at 21 days of life, the mean areas of neuronal nuclei and cytoplasm were significantly decreased within the PVN and VMN, and the mean area of neuronal cytoplasm was also decreased in the ARC (Plagemann et al. 1999b). In the adult offspring of the mildly diabetic pregnant dam, there was a significant increase in the number of NPY containing neurones within the ARC (Plagemann et al. 1998, 1999c). In a study in which control rats were reared by diabetic rat dams, it was found that there were no morphometric malformations in the hypothalamic VMN, and the authors concluded that exposure to a diabetic intrauterine milieu is critical for the reorganization of the VMN in offspring of diabetic rat dams (Fahrenkrog et al. 2004). In contrast, exclusive exposure to milk from a diabetic dam resulted in an up-regulation of NPY and AgRP peptides in the ARC of the control offspring and a decreased immunostaining for both POMC and αMSH (Fahrenkrog et al. 2004). Thus there appears to be a series of critical windows both before and after birth when exposure to enhanced nutrition or to breast milk from diabetic mothers has consequences for the development of the hypothalamic appetite regulatory system that persist into postnatal life.
When rats are undernourished (50% decrease in energy intake) during the first two weeks of pregnancy and refed during the third week, the male offspring develop significant hyperphagia and obesity when maintained on a high fat diet (Jones & Friedman, 1982; Jones et al. 1984, 1996a; Anguita et al. 1993). The obesity has a delayed onset (∼50 days of age) and refeeding during the third week of pregnancy is critical for the induction of postnatal obesity (Stephens, 1980). When maternal nutrition was restricted to 30% of control intake throughout the whole of gestation, the offspring were smaller throughout postnatal life, but they had an increase in the relative mass of retroperitoneal fat at 100 days of age (Vickers et al. 2000). Food intake by the offspring of the undernourished rats (cross fostered by ad libitum fed mothers) was higher early in postnatal life, increased with increasing age and was amplified by postnatal hypercaloric nutrition (Vickers et al. 2000). It is not yet clear whether there are accompanying changes within the hypothalamic appetite regulatory network in these animals.
Mechanisms underlying the early programming of appetite in the rodent
Both mild gestational diabetes and a reduction in litter size are associated with perinatal hyperinsulinism and there is evidence that exposure to hyperinsulinaemia during fetal or early postnatal life results in increased adiposity and altered hypothalamic development (Jones et al. 1995, 1996b; Harder et al. 1998, 1999). Protein restriction maintained during gestation and lactation is associated with hypoinsulinaemia, normal leptin concentrations and an increase in NPY levels in the ARC, PVN and LHA. There are, however, fewer neurones immunopositive for NPY in the ARC of these offspring (Plagemann et al. 2000). These authors have therefore suggested that hypoplasia of neurones expressing the orexigenic peptides such as NPY is the result of perinatal hypoinsulinism whereas hyperplasia of these neurones is a consequence of perinatal hyperinsulinism.
Interestingly, a recent study has reported that neural projection pathways from the ARC are permanently disrupted in leptin deficient (Lepob/Lepob) mice and that treatment of these mice with leptin in neonatal life, but not in adult life, rescues the development of the ARC projections (Bouret et al. 2004b). These data provide direct evidence that leptin promotes formation of hypothalamic pathways that later convey leptin signals to brain regions regulating food intake and energy consumption. This developmental activity appears to be specific for ARC projections and is restricted to a neonatal window of maximum sensitivity that corresponds to a period of elevated leptin secretion. This neonatal ‘critical period’ corresponds to the period when ARC axons are guided to their targets (Bouret et al. 2004b). In rodents, the capacity of fetal adipocytes to synthesize leptin is low until relatively late in gestation; the placenta also synthesizes little if any leptin (Kawai et al. 1997; Amico et al. 1998), although there is evidence of significant transplacental transfer of maternal leptin to the fetus (Smith & Waddell 2002, 2003).
Thus it appears that glucose, insulin and leptin derived from the maternal circulation or present in her breast milk exert the dominant influence on the development of the appetite regulatory neural network and that the immediate postnatal period is of particular importance for the long-term programming of food intake in the rodent. However, the role of maternal metabolic and hormonal signals and the critical windows during which programming of appetite may occur in the litter-bearing, altricial rodent are likely to be different from those in non-litter-bearing, precocial species such as the human and sheep.
Development and programming of the appetite regulatory system in the human
In contrast to the rodent, the earliest stage that NPY immunoreactivity was found to be present in the ARC of the human hypothalamus was at 21 weeks gestation and furthermore there were already projections from the ARC to the PVN at this stage of pregnancy (Koutcherov et al. 2002). In pregnancies complicated by maternal diabetes, the fetus is hyperglycaemic and hyperinsulinaemic, and cord blood leptin concentrations are also increased in parallel with increases in infant adiposity (Koistinen et al. 1997; Matsuda et al. 1997; Jaquet et al. 1998; Shekhawat et al. 1998; Cetin et al. 2000; Tapanainen et al. 2001). Whilst plasma leptin concentrations are low in growth restricted infants at birth, they increase to become higher in these infants at 1 year of age when compared to their normal birth weight counterparts (Jaquet et al. 1999). It has also been demonstrated that people with low birth weight also go on to have higher leptin concentrations in adult life when compared to individuals at the same BMI but with a higher birth weight (Phillips et al. 1999). Furthermore, the ratio of leptin to fat mass was significantly greater in the children who had received a nutrient enriched preterm formula than in those who received a standard formula or banked breast milk (Singhal et al. 2002). These authors concluded that programming of relative leptin concentrations by early diet may be one mechanism that links early nutrition with later obesity (Singhal et al. 2002). In order to determine the role of prenatal nutrient and hormonal signals in the programmed development of the appetite regulatory neural network it is helpful to work with an animal model, such as the sheep, in which there is prenatal development of the neural network and in which fat is deposited before birth as in the human.
Programming of the appetite regulatory system in the sheep
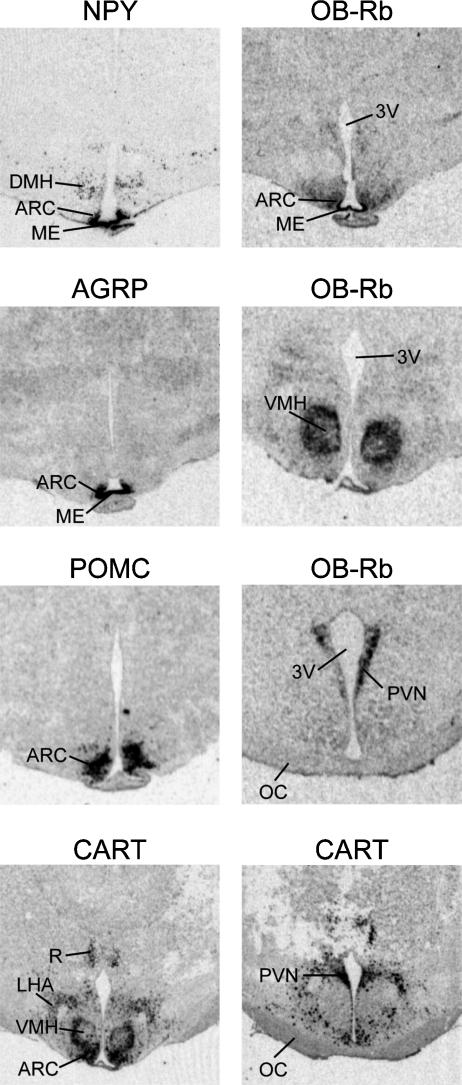
We have previously reported that genes for the appetite regulating neuropeptides NPY, AgRP, POMC and CART are each highly expressed in the ventromedial portion of the ARC of the fetal sheep hypothalamus by 110 days gestation (term = 147 ± 3 days gestation), which is consistent with their pattern of expression in the adult sheep hypothalamus (Adam et al. 2002; Mühlhäusler et al. 2004) (Fig. 1). Furthermore, and in contrast to the rodent, NPY projections are also present in the fetal PVN during late gestation (Warnes et al. 1998). Messenger RNA for the long form of the leptin receptor (OB-Rb) is also expressed in both the ARC and VMN of the fetal sheep, and to a lesser extent in the DMN, consistent with the reported pattern of expression in the adult sheep (Williams et al. 1999; Mühlhäusler et al. 2004) (Fig. 1). Whilst the sites of OB-Rb expression were similar in the fetal and adult sheep, there were differences in the relative intensity of hybridization within these hypothalamic nuclei. Specifically, the intensity of OB-Rb mRNA expression was higher in the VMN compared to the ARC in fetal sheep whereas in the adult hypothalamus, the ARC is the predominant site (Mühlhäusler et al. 2004). Thus leptin may play a different role as a signal of energy balance before birth, compared to adult life. In the adult rodent, the VMN is an important site for the regulation of thermogenesis in the brown adipose tissue (Cannon & Nedergaard, 2004), and it is possible to speculate that the higher level of OB-Rb expression in the fetal VMN indicates that leptin has a greater role in the regulation of the thermogenic activity of brown adipose tissue, rather than ‘energy intake’ during the perinatal period.
Figure 1. Autoradiographic images of coronal sections through fetal sheep hypothalamus at 110 days gestation (term ≈ 147 days) showing gene expression for NPY, AgRP, POMC, CART and leptin receptor (OB-Rb).
3V, third ventricle; ARC, arcuate nucleus; ME, median eminence; VMH, ventromedial hypothalamus; DMH, dorsomedial hypothalamus; PVN, paraventricular nucleus; R, reuniens thalamic nucleus; OC, optic chiasm. Scale bar = 1.5 mm. (From Mühlhäusler et al. 2004, with permission from Blackwell Publishing Ltd.)
In a recent study we have demonstrated that an intrafetal infusion of glucose between 130 and 140 days gestation resulted in a significant increase in POMC mRNA in the ARC of the fetal sheep hypothalamus (Mühlhäusler et al. 2005). This occurred in the absence of an increase in circulating leptin, indicating that POMC mRNA expression in the fetal hypothalamus may be responsive to increases in glucose or insulin, acting either alone or in combination. Interestingly, in this study, POMC mRNA expression in the ARC was related directly to the total expression of OB-Rb within the ARC and VMN of the fetal hypothalamus (Mühlhäusler et al. 2005). Thus glucose may act to stimulate a population of POMC containing neurones within the ARC which coexpress OB-Rb. In glucose infused fetuses, there was also a direct relationship between the expression of CART mRNA outside the ARC (i.e. within the VMN, LHA and PVN) and expression of POMC mRNA within the ARC, which may indicate a role for CART in ‘second order’ neurones as part of a neural network within the fetal hypothalamus activated by an increase in nutrient supply.
Interestingly, there was no effect of intrafetal glucose infusion on the expression of the orexigenic neuropeptides NPY and AgRP in the fetal sheep hypothalamus (Mühlhäusler et al. 2005). This is surprising given that circulating glucose and insulin concentrations in the fetus are relatively low compared with those measured in adult life and that fetal hypothalamic NPY content is increased following maternal undernutrition in sheep (Warnes et al. 1998). Fetal hypothalamic expression of NPY and AgRP may therefore be relatively insensitive to an increase in fetal glucose or insulin concentrations and indeed the preservation of orexigenic drive may be an important survival strategy for the neonate immediately after birth.
In sheep, leptin is synthesized in fetal adipose tissue and is present in the fetal circulation in lower concentrations than in the maternal circulation through late gestation (Yuen et al. 1999, 2002; Chen et al. 2000; Devaskar et al. 2002; Ehrhardt et al. 2002; Mühlhäusler et al. 2002, 2003). As the sheep placenta expresses the leptin receptor gene (Thomas et al. 2001) and maternal and fetal plasma leptin concentrations are positively correlated throughout late gestation (Yuen et al. 2002), it is possible that the placental leptin receptor may mediate the uptake of leptin from the maternal into the fetal circulation. Fetal adipocytes also contain larger or dominant lipid locules and there is a direct relationship between the relative mass of the ‘unilocular’ component of perirenal and interscapular fat and the circulating leptin concentrations in fetuses of well nourished pregnant ewes (Mühlhäusler et al. 2002). This suggests that that circulating leptin concentrations may be a signal of the unilocular component of fat in fetal life, rather than total fat mass as it is in the neonate and adult. Intrafetal leptin infusion in the presence of normoglycaemia and normoinsulinaemia results in a decrease in the proportion and relative mass of unilocular tissue in the perirenal adipose depot and a decrease in the relative abundance of leptin mRNA in perirenal adipose tissue in fetal sheep (Yuen et al. 2003). The precise site of this action of leptin, either within the fetal hypothalamus or peripherally within the adipoinsular axis remains to be determined.
Whilst there is evidence that the hypothalamic neural network that regulates appetite in adult life is present in the fetus and is responsive to changes in circulating signals of nutrition before birth, there is relatively little information on whether this axis may be programmed prenatally in the sheep. It has been demonstrated that low birth weight lambs have a higher relative voluntary food intake during the early postnatal period and are fatter at body weights up to 20 kg when compared with lambs with normal birth weights (Greenwood et al. 1998). One possibility is that this relative hyperphagia is, at least in part, a result of early programming of the appetite regulatory neuropeptide network.
Perspective
A series of studies have provided significant evidence that changes in perinatal nutrition programme the development of the hypothalamic neural network that regulates appetite in adult life. These studies have been primarily in the rodent utilizing maternal overnutrition or undernutrition imposed at different stages of pregnancy and beyond, mapping of neuropeptide localization and activity and using appropriate null mutant models. Such research has provided a neuroanatomical and functional framework for the oft mooted hypothalamic body weight ‘set point’ hypothesis (Elmquist & Flier, 2004) and this can now be interrogated experimentally to determine the extent to which there could be perinatal programming of such a set point by exposure to relative over- or undernutrition in the human fetus. Whilst the rodent offers significant advantages in terms of a short gestation and the availability of useful transgenic and null mutant models, there are clear advantages to using an animal model more akin to the human, in which all components of the ‘fat–brain axis’ develop before birth, such as the sheep. Use of this model will allow a definition of the role(s) played by the ‘appetite regulatory’ peptides before birth and whether there are critical prenatal windows for the programming of postnatal appetite. In the face of the global obesity epidemic, there is a potential for an intergenerational cycle of obesity as women enter pregnancy with a higher BMI (Kral, 2004). Thus, in contrast to the general focus of the ‘early origins of adult disease’ field on prenatal growth restriction, there is an increasing impetus to define the short and longer term consequences of exposure of the hypothalamic neural network which controls energy balance to maternal and hence fetal nutritional excess.
References
- Adam CL, Archer ZA, Findlay PA, Thomas L, Marie M. Hypothalamic gene expression in sheep for cocaine- and amphetamine-regulated transcript, pro-opiomelanocortin, neuropeptide Y, agouti-related peptide and leptin receptor and responses to negative energy balance. Neuroendocrinology. 2002;75:250–256. doi: 10.1159/000054716. 10.1159/000054716. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. 10.1016/S1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Allen JM, McGregor GP, Woodhams PL, Polak JM, Bloom SR. Ontogeny of a novel peptide, neuropeptide Y (NPY) in rat brain. Brain Res. 1984;303:197–200. doi: 10.1016/0006-8993(84)90230-0. 10.1016/0006-8993(84)90230-0. [DOI] [PubMed] [Google Scholar]
- Amico JA, Thomas A, Crowley RS, Burmeister LA. Concentrations of leptin in the serum of pregnant, lactating, and cycling rats and of leptin messenger ribonucleic acid in rat placental tissue. Life Sci. 1998;63:1387–1395. doi: 10.1016/s0024-3205(98)00405-6. 10.1016/S0024-3205(98)00405-6. [DOI] [PubMed] [Google Scholar]
- Anguita RM, Sigulem DM, Sawaya AL. Intrauterine food restriction is associated with obesity in young rats. J Nutr. 1993;123:1421–1428. doi: 10.1093/jn/123.8.1421. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood–brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004a;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004b;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Kjos SL. Gestational diabetes: Risk or myth? J Clin Endocrinol Metab. 1999;84:1854–1857. doi: 10.1210/jcem.84.6.5714. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Burn P. Strategies and potential molecular targets for obesity treatment. Science. 1998;280:1383–1387. doi: 10.1126/science.280.5368.1383. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cetin I, Morpurgo PS, Radelli T, Taricco E, Cortelazzi D, Bellotti M, Pardi G, Beck-Peccoz P. Fetal plasma leptin concentrations: Relationship with different intrauterine growth patterns from 19 weeks to term. Pediatr Res. 2000;48:646–651. doi: 10.1203/00006450-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Chen X, Lin J, Hausman DB, Martin RJ, Dean RG, Hausman GJ. Alterations in fetal adipose tissue leptin expression correlate with the development of adipose tissue. Biol Neonate. 2000;78:41–47. doi: 10.1159/000014245. [DOI] [PubMed] [Google Scholar]
- Chessler SD, Fujimoto WY, Shofer JB, Boyko EJ, Weigle DS. Increased plasma leptin levels are associated with fat accumulation in Japanese Americans. Diabetes. 1998;47:239–243. doi: 10.2337/diab.47.2.239. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996a;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascerio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus and obesity in US men. Circulation. 1996b;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Davidowa H, Li Y, Plagemann A. Hypothalamic ventromedial and arcuate neurons of normal and postnatally overnourished rats differ in their responses to melaninconcentrating hormone. Regul Pept. 2002;108:103–111. doi: 10.1016/s0167-0115(02)00153-2. 10.1016/S0167-0115(02)00153-2. [DOI] [PubMed] [Google Scholar]
- Davidowa H, Li Y, Plagemann A. Altered responses to orexigenic (AGRP, MCH) and anorexigenic (alpha-MSH, CART) neuropeptides of paraventricular hypothalamic neurons in early postnatally overfed rats. Eur J Neurosci. 2003;18:613–621. doi: 10.1046/j.1460-9568.2003.02789.x. 10.1046/j.1460-9568.2003.02789.x. [DOI] [PubMed] [Google Scholar]
- Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport. 2000;21:2795–2798. doi: 10.1097/00001756-200008210-00037. [DOI] [PubMed] [Google Scholar]
- Davidowa H, Plagemann A. Inhibition by insulin of hypothalamic VMN neurons in rats overweight due to postnatal overfeeding. Neuroreport. 2001;12:3201–3204. doi: 10.1097/00001756-200110290-00012. 10.1097/00001756-200110290-00012. [DOI] [PubMed] [Google Scholar]
- Devaskar SU, Anthony R, Hay W., Jr Ontogeny and insulin regulation of fetal ovine white adipose tissue leptin expression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R431–R438. doi: 10.1152/ajpregu.2002.282.2.R431. [DOI] [PubMed] [Google Scholar]
- Dorner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res. 1994;26:213–221. doi: 10.1055/s-2007-1001668. [DOI] [PubMed] [Google Scholar]
- Ehrhardt RA, Bell AW, Boisclair YR. Spatial and developmental regulation of leptin in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1628–R1635. doi: 10.1152/ajpregu.00750.2001. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Flier JS. The fat-brain axis enters a new dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, Plagemann A. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. 2004;134:648–654. doi: 10.1093/jn/134.3.648. [DOI] [PubMed] [Google Scholar]
- Fall CHD, Osmond C, Barker DJP, Clark PMS, Hales CN, Stirling Y, Meade TW. Fetal and infant growth and cardiovascular risk factors in women. Br Med J. 1995;310:428–432. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. J Am Med Assoc. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep. I. Body growth and composition, and some aspects of energetic efficiency. J Anim Sci. 1998;76:2354–2367. doi: 10.2527/1998.7692354x. [DOI] [PubMed] [Google Scholar]
- Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. 10.1016/S0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Hales CN, Desai M, Ozanne SE. The Thrifty Phenotype hypothesis: how does it look after 5 years? Diabet Med. 1997;14:189–195. doi: 10.1002/(SICI)1096-9136(199703)14:3<189::AID-DIA325>3.0.CO;2-3. 10.1002/(SICI)1096-9136(199703)14:3<189::AID-DIA325>3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Hales CN, Ozanne SE. For debate: Fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia. 2003;46:1013–1019. doi: 10.1007/s00125-003-1131-7. 10.1007/s00125-003-1131-7. [DOI] [PubMed] [Google Scholar]
- Harder T, Plagemann A, Rohde W, Dorner G. Syndrome X-like alterations in adult female rats due to neonatal insulin treatment. Metabolism. 1998;47:855–862. doi: 10.1016/s0026-0495(98)90126-3. 10.1016/S0026-0495(98)90126-3. [DOI] [PubMed] [Google Scholar]
- Harder T, Rake A, Rohde W, Doerner G, Plagemann A. Overweight and increased diabetes susceptibility in neonatally insulin-treated adult rats. Endocr Regul. 1999;33:25–31. [PubMed] [Google Scholar]
- Heidel E, Plagemann A, Davidowa H. Increased response to NPY of hypothalamic VMN neurons in postnatally overfed juvenile rats. Neuroreport. 1999;10:1827–1831. doi: 10.1097/00001756-199906230-00005. [DOI] [PubMed] [Google Scholar]
- Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J Physiol. 1999;514:617–627. doi: 10.1111/j.1469-7793.1999.617ad.x. 10.1111/j.1469-7793.1999.617ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holemans K, Verhaeghe J, Dequeker J, Van Assche FA. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J Soc Gynecol Invest. 1996;3:71–77. doi: 10.1016/1071-5576(95)00046-1. 10.1016/1071-5576(95)00046-1. [DOI] [PubMed] [Google Scholar]
- James WP. The epidemiology of obesity. Ciba Found Symp. 1996;201:1–11. doi: 10.1002/9780470514962.ch1. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: Effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83:1243–1246. doi: 10.1210/jcem.83.4.4731. 10.1210/jc.83.4.1243. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Leger J, Tabone MD, Czernichow P, Levy Marchal C. High serum leptin concentrations during catch-up growth of children born with intrauterine growth retardation. J Clin Endocrinol Metab. 1999;84:1949–1953. doi: 10.1210/jcem.84.6.5744. 10.1210/jc.84.6.1949. [DOI] [PubMed] [Google Scholar]
- Jones AP, Assimon SA, Friedman MI. The effect of diet on food intake and adiposity in rats made obese by gestational undernutrition. Physiol Behav. 1996a;37:381–386. doi: 10.1016/0031-9384(86)90194-0. 10.1016/0031-9384(86)90194-0. [DOI] [PubMed] [Google Scholar]
- Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- Jones AP, Olster DH, States B. Maternal insulin manipulations in rats organize body weight and noradrenergic innervation of the hypothalamus in gonadally intact male offspring. Brain Res Dev Brain Res. 1996b;97:16–21. doi: 10.1016/s0165-3806(96)00128-9. 10.1016/S0165-3806(96)00128-9. [DOI] [PubMed] [Google Scholar]
- Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG. Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res Dev Brain Res. 1995;88:127–131. doi: 10.1016/0165-3806(95)00078-r. 10.1016/0165-3806(95)00078-R. [DOI] [PubMed] [Google Scholar]
- Jones AP, Simson EL, Friedman MI. Gestational undernutrition and the development of obesity in rats. J Nutr. 1984;114:1484–1492. doi: 10.1093/jn/114.8.1484. [DOI] [PubMed] [Google Scholar]
- Kagotani Y, Hashimoto T, Tsuruo Y, Kawano H, Daikoku S, Chihara K. Development of the neuronal system containing neuropeptide Y in the rat hypothalamus. Int J Dev Neurosci. 1989;7:359–374. doi: 10.1016/0736-5748(89)90057-9. 10.1016/0736-5748(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Kawai M, Yamaguchi M, Murakami T, Shima K, Murata Y, Kishi K. The placenta is not the main source of leptin production in pregnant rat: Gestational profile of leptin in plasma and adipose tissues. Biochem Biophys Res Commun. 1997;240:798–802. doi: 10.1006/bbrc.1997.7750. 10.1006/bbrc.1997.7750. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun. 1996;224:522–527. doi: 10.1006/bbrc.1996.1059. 10.1006/bbrc.1996.1059. [DOI] [PubMed] [Google Scholar]
- Koistinen HA, Koivisto VA, Andersson S, Karonen SL, Kontula K, Oksanen L, Teramo KA. Leptin concentration in cord blood correlates with intrauterine growth. J Clin Endocrinol Metab. 1997;82:3328–3330. doi: 10.1210/jcem.82.10.4291. 10.1210/jc.82.10.3328. [DOI] [PubMed] [Google Scholar]
- Koutcherov Y, Mai JK, Ashwell KW, Paxinos G. Organization of human hypothalamus in fetal development. J Comp Neurol. 2002;446:310–324. doi: 10.1002/cne.10175. [DOI] [PubMed] [Google Scholar]
- Kral JG. Preventing and treating obesity in girls and young women to curb the epidemic. Obes Res. 2004;12:1539–1546. doi: 10.1038/oby.2004.193. [DOI] [PubMed] [Google Scholar]
- Law CM, Barker DJP, Osmond C, Fall CHD, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Community Health. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Plagemann A, Davidowa H. Increased inhibition by agouti-related peptide of ventromedial hypothalamic neurons in rats overweight due to early postnatal overfeeding. Neurosci Lett. 2002;330:33–36. doi: 10.1016/s0304-3940(02)00722-x. 10.1016/S0304-3940(02)00722-X. [DOI] [PubMed] [Google Scholar]
- Lindroos A, Lissner L, Carlsson B, Carlsson L, Torgerson J, Karlsson C, Stenlof K, Sjostrom L. Familial predisposition for obesity may modify the predictive value of serum leptin concentrations for long-term weight change in obese women. Am J Clin Nutr. 1998;67:1119–1123. doi: 10.1093/ajcn/67.6.1119. [DOI] [PubMed] [Google Scholar]
- Lissner L, Karlsson C, Lindroos AK, Sjostrom L, Carlsson B, Carlsson L, Bengtsson C. Birth weight, adulthood BMI, and subsequent weight gain in relation to leptin levels in Swedish women. Obesity Res. 1999;7:150–154. doi: 10.1002/j.1550-8528.1999.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young adult men – a prospective twin study. Int J Obes Relat Metab Disord. 2001;25:1537–1545. doi: 10.1038/sj.ijo.0801743. 10.1038/sj.ijo.0801743. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young women: a prospective twin study. Am J Clin Nutr. 2002;75:676–682. doi: 10.1093/ajcn/75.4.676. [DOI] [PubMed] [Google Scholar]
- Maffeis C, Micciolo R, Must A, Zaffanello M, Pinelli L. Parental and perinatal factors associated with childhood obesity in north-east Italy. Int J Obes Relat Metab Disord. 1994;18:301–305. [PubMed] [Google Scholar]
- Malina RM, Katzmarzyk PT, Beunen G. Birth weight and its relationship to size attained and relative fat distribution at 7–12 years of age. Obes Res. 1996;4:385–390. doi: 10.1002/j.1550-8528.1996.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Yokota I, Iida M, Murakami T, Naito EITOM, Shima K, Kuroda Y. Serum leptin concentration in cord blood: relationship to birth weight and gender. J Clin Endocrinol Metab. 1997;82:1642–1644. doi: 10.1210/jcem.82.5.4063. 10.1210/jc.82.5.1642. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Mühlhäusler BS, Duffield JA, Yuen BS. Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proc Nutrition Soc. 2004;63:405–412. doi: 10.1079/pns2004370. 10.1079/PNS2004370. [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Adam CL, Marrocco EM, Findlay PA, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J Physiol. 2005;565:185–195. doi: 10.1113/jphysiol.2004.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlhäusler BS, McMillen IC, Rouzaud G, Findlay PA, Marrocco EM, Rhind SM, Adam CL. Appetite regulatory neuropeptides are expressed in the sheep hypothalamus before birth. J Neuroendocrinol. 2004;16:502–507. doi: 10.1111/j.1365-2826.2004.01197.x. 10.1111/j.1365-2826.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biol Reprod. 2002;67:493–499. doi: 10.1095/biolreprod67.2.493. [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Roberts CT, Yuen BSJ, Marrocco E, Budge H, Symonds ME, McFarlane JR, Kauter KG, Stagg P, Pearse JK, McMillen IC. Determinants of fetal leptin synthesis, fat mass, and circulating leptin concentrations in well-nourished ewes in late pregnancy. Endocrinology. 2003;144:4947–4954. doi: 10.1210/en.2003-0555. 10.1210/en.2003-0555. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. J Am Med Assoc. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Oh W, Gelardi NL, Cha CJ. Maternal hyperglycemia in pregnant rats: its effect on growth and carbohydrate metabolism in the offspring. Metabolism. 1988;37:1146–1151. doi: 10.1016/0026-0495(88)90192-8. 10.1016/0026-0495(88)90192-8. [DOI] [PubMed] [Google Scholar]
- Okosun IS, Liao Y, Rotimi CN, Dever GE, Cooper RS. Impact of birth weight on ethnic variations in subcutaneous and central adiposity in American children aged 5–11 years. A study from the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2000;24:479–484. doi: 10.1038/sj.ijo.0801182. 10.1038/sj.ijo.0801182. [DOI] [PubMed] [Google Scholar]
- Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol Regul Integr Comp Physiol. 1978;235:R141–R144. doi: 10.1152/ajpregu.1978.235.3.R141. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. Br Med J. 2001;323:1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DIW, Fall CHD, Cooper C, Norman RJ, Robinson JS, Owens PC. Size at birth and plasma leptin concentrations in adult life. Int J Obes Relat Metab Disord. 1999;23:1025–1029. doi: 10.1038/sj.ijo.0801050. 10.1038/sj.ijo.0801050. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Janert U, Rake A, Rittel F, Rohde W, Dorner G. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev Neurosci. 1999b;21:58–67. doi: 10.1159/000017367. 10.1159/000017367. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Melchior K, Rake A, Rohde W, Dorner G. Elevation of hypothalamic neuropeptide Y-neurons in adult offspring of diabetic mother rats. Neuroreport. 1999c;10:3211–3216. doi: 10.1097/00001756-199910190-00016. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Melchior K, Rittel F, Rohde W, Dorner G. Hypothalamic insulin and neuropeptide Y in the offspring of gestational diabetic mother rats. Neuroreport. 1998;9:4069–4073. doi: 10.1097/00001756-199812210-00012. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999d;836:146–155. doi: 10.1016/s0006-8993(99)01662-5. 10.1016/S0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999a;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides. 2000;34:1–6. doi: 10.1054/npep.1999.0778. 10.1054/npep.1999.0778. [DOI] [PubMed] [Google Scholar]
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- Reaven G. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Schwartz MW. Brain pathways controlling food intake and body weight. Exp Biol Med. 2001;226:978–981. doi: 10.1177/153537020122601103. [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Garland JS, Shivpuri C, Mick GJ, Sasidharan P, Pelz CJ, McCormick KL. Neonatal cord blood leptin: its relationship to birth weight, body mass index, maternal diabetes, and steroids. Pediatr Res. 1998;43:338–343. doi: 10.1203/00006450-199803000-00005. [DOI] [PubMed] [Google Scholar]
- Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40(Suppl. 2):121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- Singhal A, Farooqi IS, O'Rahilly S, Cole TJ, Fewtrell M, Lucas A. Early nutrition and leptin concentrations in later life. Am J Clin Nutr. 2002;75:993–999. doi: 10.1093/ajcn/75.6.993. [DOI] [PubMed] [Google Scholar]
- Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–730. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Leptin receptor expression in the rat placenta: changes in Ob-Ra, Ob-Rb, and Ob-Re with gestational age and suppression by glucocorticoids. Biol Reprod. 2002;67:1204–1210. doi: 10.1095/biolreprod67.4.1204. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144:3024–3030. doi: 10.1210/en.2003-0145. 10.1210/en.2003-0145. [DOI] [PubMed] [Google Scholar]
- Stephens DN. Growth and development of dietary obesity in adulthood of rats which have been undernourished during development. Br J Nutr. 1980;44:215–227. doi: 10.1079/bjn19800034. [DOI] [PubMed] [Google Scholar]
- Tapanainen P, Leinonen E, Ruokonen A, Knip M. Leptin concentrations are elevated in newborn infants of diabetic mothers. Horm Res. 2001;55:185–190. doi: 10.1159/000049993. 10.1159/000049993. [DOI] [PubMed] [Google Scholar]
- Thomas L, Wallace JM, Aitken RP, Mercer JG, Trayhurn P, Hoggard N. Circulating leptin during ovine pregnancy in relation to maternal nutrition, body composition and pregnancy outcome. J Endocrinol. 2001;169:465–476. doi: 10.1677/joe.0.1690465. 10.1677/joe.0.1690465. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- Warnes KE, Morris MJ, Symonds ME, Phillips ID, Clarke IJ, Owens JA, McMillen IC. Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus. J Neuroendocrinol. 1998;10:51–57. doi: 10.1046/j.1365-2826.1998.00172.x. 10.1046/j.1365-2826.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Adam CL, Mercer JG, Moar KM, Slater D, Hunter L, Findlay PA, Hoggard N. Leptin receptor and neuropeptide Y gene expression in the sheep brain. J Neuroendocrinol. 1999;11:165–169. doi: 10.1046/j.1365-2826.1999.00293.x. 10.1046/j.1365-2826.1999.00293.x. [DOI] [PubMed] [Google Scholar]
- Woodhams PL, Allen YS, McGovern J, Allen JM, Bloom SR, Balazs R, Polak J. Immunohistochemical analysis of the early ontogeny of the neuropeptide Y system in rat brain. Neuroscience. 1985;15:173–202. doi: 10.1016/0306-4522(85)90131-9. 10.1016/0306-4522(85)90131-9. [DOI] [PubMed] [Google Scholar]
- Yuen BSJ, McMillen IC, Symonds ME, Owens PC. Abundance of leptin mRNA in fetal adipose tissue is related to fetal body weight. J Endocrinol. 1999;163:R11–R14. doi: 10.1677/joe.0.163r011. 10.1677/joe.0.163R011. [DOI] [PubMed] [Google Scholar]
- Yuen BSJ, Owens PC, McFarlane JR, Symonds ME, Edwards LJ, Kauter KG, McMillen IC. Circulating leptin concentrations are positively related to leptin messenger RNA expression in the adipose tissue of fetal sheep in the pregnant ewe fed at or below maintenance energy requirements during late gestation. Biol Reprod. 2002;67:911–916. doi: 10.1095/biolreprod.101.002931. 10.1095/biolreprod.101.002931. [DOI] [PubMed] [Google Scholar]
- Yuen BSJ, Owens PC, Mühlhäusler BS, Roberts CT, Symonds ME, Keisler DH, McFarlane JR, Kauter KG, Evens Y, McMillen IC. Leptin alters the structural and functional characteristics of adipose tissue before birth. FASEB J. 2003;17:1102–1104. doi: 10.1096/fj.02-0756fje. [DOI] [PubMed] [Google Scholar]