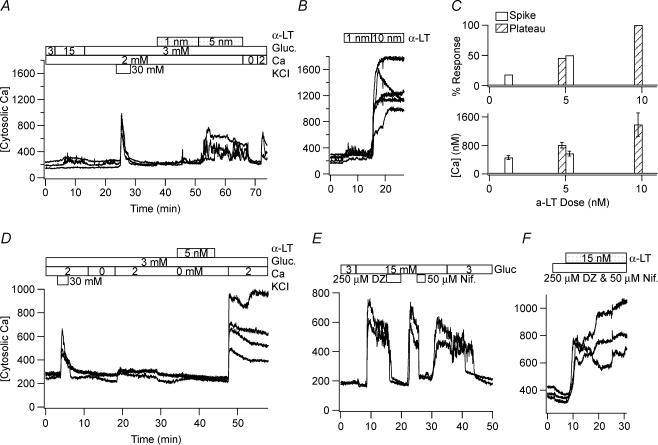
Figure 1. Nanomolar concentrations of α-LT cause large changes in [Ca2+]i of human and mouse islet cells that are dependent on the presence of an adequate [Ca2+]o.
A and B, 5 nm and 10 nm α-LT, respectively, raise the estimated [Ca2+]i in representative glucose- and KCl-responsive human islet cells from baseline level of ∼200 nm to a maximum level of ∼450 nm and ∼800 nm, respectively, while 1 nm α-LT generates brief spikes in a minority of cells. C, top histograms show the percentage of human cells responding to α-LT with an increase in [Ca2+]i found by those displaying transient spikes (filled bar) versus a more sustained plateau (striped bar). C, bottom histograms show the corresponding average of maximum [Ca2+]i±s.d. of the responding cells. D, α-LT binds equally well in the absence of Ca2+o. E and F, the combination of 250 μm diazoxide (an opener of ATP-dependent K+ channels), which hyperpolarizes β-cells, and 50 μm nifedipine (a blocker of L-type voltage-dependent Ca2+ channels), which blocks depolarization-induced electrical activity, does not militate the effect of α-LT on [Ca2+]i while either drug alone blocks a glucose-induced rise in [Ca2+]i.