Abstract
Brainstem 5-hydroxytryptamine (5-HT, serotonin)-containing neurones modulate cardiovascular reflex responses but the differing roles of the many 5-HT receptors have not been thoroughly investigated. The present experiments on anaesthetized rats investigated the role of 5-HT3 receptors in modulating vagal afferent evoked activity of nucleus tractus solitarius (NTS) neurones. Recordings were made from 301 NTS neurones receiving an input at long (> 20 ms) minimum onset latency from stimulation of the vagus nerve. These included 140 neurones excited by activating non-myelinated cardiopulmonary afferents by right atrial injection of phenylbiguanide (PBG). Ionophoretic application of PBG, a highly selective 5-HT3 receptor agonist, significantly increased activity (from 2.4 ± 0.4 to 5.5 ± 0.8 spikes s−1) in 96 of 106 neurones tested and in all 17 neurones tested the increase in activity (3.4 ± 1.1 to 7.0 ± 1.9 spikes s−1) was significantly attenuated (3.0 ± 0.9 to 3.8 ± 1.1 spikes s−1) by the selective 5-HT3 receptor antagonist granisetron. Ionophoretic application of PBG potentiated responses to vagus nerve and cardiopulmonary afferent stimulation, and granisetron significantly attenuated this cardiopulmonary input (20.2 ± 5.7 to 10.6 ± 4.1 spikes burst−1) in 9 of 10 neurones tested. Ionophoretic application of AMPA and NMDA also excited NTS neurones and these excitations could be selectively antagonized by the non-NMDA and NMDA receptor antagonists DNQX and AP-5, respectively. At these selective currents, DNQX and AP-5 also attenuated PBG- and cardiopulmonary input-evoked increases in NTS activity. These data are consistent with the hypothesis that vagal inputs, including non-myelinated cardiopulmonary inputs to the NTS, utilize a 5-HT-containing pathway which activates 5-HT3 receptors. This excitatory response to 5-HT3 receptor activation may be partly a direct postsynaptic action but part may also be due to facilitation of the release of glutamate which in turn acts on either non-NMDA or NMDA receptors to evoke excitation.
The central nervous system is involved in cardiovascular homeostasis by constantly monitoring information from sensory receptors such as the arterial baroreceptors and chemoreceptors, and receptors in the cardiopulmonary region. Afferent fibres carrying this information terminate in the nucleus tractus solitarius (NTS, see Jordan & Spyer, 1986). Recent neuroanatomical and electrophysiological studies have shown that the sensory information is processed in the NTS by extensive intrinsic circuitry – activation of different sensory inputs may affect different groups of NTS cells, but also many cells can receive convergent input from two or more sets of afferents. Additionally, the NTS is innervated and modulated by many other regions of the CNS (see Taylor et al. 1999; Jordan, 2001 for review). Numerous neurotransmitters have been suggested to be involved in the neural pathways mediating cardiovascular control (see Lawrence & Jarrot, 1996) and work from our and other laboratories over the last decade has demonstrated that 5-hydroxytryptamine (5-HT) has important influences at multiple sites responsible for controlling autonomic outflow (see Ramage, 2001; Jordan, 2005). These include the NTS which is densely innervated by 5-HT-containing fibres (Steinbusch, 1981; Sykes et al. 1994).
Application of 5-HT to the NTS has been reported to have diverse cardiovascular effects, evoking falls in blood pressure and heart rate (Laguzzi et al. 1984) or rises (Merahi et al. 1992) in blood pressure. This may not be surprising since this is a functionally heterogenous nucleus and many of the 14 identified 5-HT receptor subtypes have been found here. Binding sites or mRNA for 5-HT1A and 5-HT1B (Manaker & Verderame, 1990), 5-HT2A, 5-HT2C (Pompeiano et al. 1994), 5-HT3 (Steward et al. 1993; Miquel et al. 2002), 5-ht5A (Oliver et al. 2000) and 5-HT7 receptors (Gustafson et al. 1996) have been localized here. The 5-HT-evoked hypotension appears to involve both 5-HT1A (Itoh & Bunag, 1991) and 5-HT2 (N'Diaye et al. 2001) receptors whereas the hypertension seems to involve 5-HT3 receptors since it was mimicked by NTS microinjection of 5-HT3 receptor agonists and was attenuated by selective antagonists (Merahi et al. 1992; Bonagamba et al. 2000). In addition to their effects on baseline cardiovascular variables 5-HT receptors can also modulate the effectiveness of cardio-respiratory reflexes. For example, the reflex bradycardia produced by stimulating cardiopulmonary afferents in rats was attenuated by intracisternal application of 5-HT1A receptor antagonists (Bogle et al. 1990) and similar application of 5-HT7 receptor antagonists attenuated vagal bradycardias evoked by stimulating cardiopulmonary, arterial baroreceptor and chemoreceptor afferents suggesting a facilitatory role for these receptors (Kellett et al. 2005). Similarly, some evidence suggests that 5-HT3 receptors may also be involved in the bradycardias evoked by stimulating upper airway (Dando et al. 1995) or cardiopulmonary afferents (Pires et al. 1998) since intracisternal administration of granisetron, a selective 5-HT3 receptor antagonist attenuated these bradycardias. However, stimulating 5-HT3 receptors by NTS microinjection of 5-HT3 receptor agonists has also been reported to attenuate reflex bradycardias evoked by stimulating arterial baroreceptors (Merahi et al. 1992; Callera et al. 1997; Bonagamba et al. 2000), carotid chemoreceptors (Sévoz et al. 1997; Callera et al. 1997) and cardiopulmonary afferents (Sévoz et al. 1996; Leal et al. 2001).
Within the NTS application of 5-HT to neurones by ionophoresis in vivo has variable effects depending on the amount applied and the individual neurones (Wang et al. 1997; Jordan, 2005). This probably reflects the profile and/or location of different 5-HT receptors on the NTS neurones and confirms in vitro studies using brainstem slices where application of 5-HT evoked both excitations and inhibitions. Inhibitory responses were attributed to activation of 5-HT1 receptors (Feldman, 1995) whilst the excitations were due to activation of 5-HT2 and 5-HT3 receptors. Activation of 5-HT3 receptors had both pre- and postsynaptic effects, increasing the amplitude and frequency of spontaneous postsynaptic potentials (PSPs) in addition to a direct postsynaptic depolarization (Glaum et al. 1992; Brooks & Albert, 1995). This would be consistent with binding data demonstrating that vagotomy reduces substantially the number of 5-HT3 binding sites within the NTS (Pratt & Bowery, 1989; Kidd et al. 1993; Leslie et al. 1994) and ultrastructural data showing the presence of immunoreactivity for the 5-HT3A receptor subunit on small non-myelinated axons and axon terminals and on somatodendritic profiles within the medial NTS (Huang et al. 2004). In previous publications it was demonstrated that 5-HT3 receptor activation excited vagal preganglionic neurones in the dorsal vagal nucleus and which was mediated, at least in part, by facilitation of glutamate release from presynaptic sites (Wang et al. 1996, 1998). Apart from one abstract demonstrating that the selective 5-HT3 receptor antagonist granisetron attenuated both vagal-evoked and spontaneous discharge of NTS neurones (Ramage & Mifflin, 1998), there is no information in vivo on the role of central 5-HT3 receptors in processing reflex inputs within the NTS. The present study was performed to investigate the effects of 5-HT3 receptors on the spontaneous activity of NTS neurones, their vagal- and cardiopulmonary afferent-evoked activity, and to assess the role of glutamate receptors in these responses.
Preliminary accounts of some of this work have been published previously (Jeggo et al. 2000, 2001; Kellett et al. 2004).
Methods
Experiments were performed on 61 male Sprague-Dawley rats (275–450 g body weight) anaesthetized with thiobutabarbitone sodium (Inactin, 120 mg kg−1, i.p.) or pentobarbitone sodium (60 mg kg−1, i.p.). These experiments were carried out under the Animals (Scientific Procedures) Act 1986. Anaesthesia was supplemented when necessary (30 mg kg−1 and 10 mg kg−1i.v., respectively). At the end of the experiment all animals were humanely killed by an overdose of pentobarbitone sodium (i.v.). A tracheotomy was performed low in the neck, and a femoral artery and vein cannulated for measurement of arterial blood pressure and administration of supplemental anaesthetic and drugs. A silicone cannula filled with phenylbiguanide (PBG; 200 μg ml−1) was advanced through the right jugular vein until it lay within the right atrium. In some animals a dual lumen cannula (filled with PBG and saline) was placed into the right atrium to allow a matched volume of saline to be injected as a volume controls. Tracheal and arterial pressures were measured with pressure transducers (model P23XL, Statham, Hato Rey, PR, USA). To record ECG two leads attached to opposite limbs were connected to an amplifier (NL 104, Neurolog, Digitimer Ltd; gain 5000) and filter unit (NL 125, Neurolog, 0.5–5 kHz). Animals were ventilated with oxygen-enriched room air using a positive pressure ventilator (Harvard Rodent ventilator, model 683) with 1 cmH2O positive end-expiratory pressure. Arterial blood samples were taken regularly and blood gases and pH monitored with a pH–blood gas analyser (model 238, Ciba Corning Diagnostics Ltd, Halstead, UK). Blood gases were maintained in the following ranges: PO2 155 ± 5 mmHg, PCO2 37 ± 1 mmHg, pH 7.35 ± 0.01 by slow i.v. infusions of sodium bicarbonate (1.0 m) and/or adjustments of the respiratory pump. Rectal temperature was monitored and maintained at 37°C with a Harvard homeothermic blanket system (Harvard Apparatus, South Natick, MA, USA). Since the rats are anaesthetized and ventilated for a prolonged period, in addition to heart rate and blood pressure, we routinely monitored and maintained respiratory variables (tracheal pressure, phrenic nerve activity and arterial blood gases) to gauge the overall physiological state of the animal and to ensure that reflexes were well maintained.
The rats were fixed in a stereotaxic frame and the phrenic nerve isolated low in the neck from a dorsolateral approach. The nerve was cut peripherally, and the proximal end desheathed and placed on bipolar platinum recording electrodes. Phrenic nerve activity was amplified (NL 104, Neurolog; gain 20 000) and filtered (NL 125, Neurolog, 0.5–5 kHz). Using the same approach, the right cervical vagus nerve was dissected free from the sympathetic trunk and placed on bipolar silver wire electrodes for electrical stimulation (50–500 μA, 1 ms, 0.3–1 Hz) with an isolated stimulator (Digitimer DS2A) triggered by a programmer (Digitimer 4030). The exposed lengths of both nerves were covered in paraffin wax and fixed in place with dental impression material (President light body dental polyvinylsiloxane – Coltene). To expose the dorsal surface of the caudal brainstem the nuchal muscles were removed from the back of the neck, the occipital bone removed and the dura overlying the brainstem cut and reflected laterally. During single cell recordings the animals were neuromuscularly blocked with decamethonium bromide (3 mg kg−1i.v. initial dose followed by 3 mg kg−1 h−1, i.v.) or a single dose of α-bungarotoxin (140 μg kg−1, i.v.). During neuromuscular blockade the depth of anaesthesia was assessed by monitoring the stability of the arterial blood pressure and heart rate and the cardiovascular responses to pinching the paws. To increase stability of the brainstem for recording, in some animals a pneumothorax was created.
Protocol
Extracellular recordings were made from neurones in the medial regions of the NTS (< 1 mm lateral to midline) within 1–2 mm caudal to obex, an area known to contain neurones receiving cardiopulmonary afferent inputs (Hines et al. 1994). We used 5- or 7-barrelled microelectrodes made from borosilicate glass (Clarke Electromedical, Reading, UK) or compound electrodes constructed by gluing a single-barrelled glass recording electrode (tip diameter ∼1 μm) to a multibarrelled glass electrode (tip diameter 3–7 μm; Wang et al. 1998; Jones et al. 2002). The recording barrel contained NaCl (0.5–4 m) whilst the other barrels contained pontamine sky blue dye and a combination of ligands for 5-HT and glutamate receptors. For the glutamate experiments PBG, AMPA and NMDA were always included in addition to one of the antagonists so that antagonist selectivity could be assessed. Neuronal recordings were amplified × 1000 to × 5000 (Axoclamp 2A, Axon Instruments, CA, USA) and filtered (0.5–5 kHz; NL 125, Neurolog). NTS neurones were identified by their orthodromic response to electrical stimulation of the cervical vagus nerve at 2 × threshold for evoking activity (Wang et al. 1997; Sévoz-Couche et al. 2000). NTS neurones receiving non-myelinated vagal input mediate a diverse range of functions. In the present study, one functional subpopulation of these neurones was identified by their response to cardiopulmonary afferent stimulation by right atrial administration of PBG (12–24 μg kg−1, 60–120 μl kg−1; Kay & Armstrong, 1990). Ligands were applied to the vicinity of the recorded neurones by ionophoresis (Neurophore, Medical Systems, Digitimer Ltd). Between drug ejection periods a retaining current of 10–15 nA was applied to each drug barrel. When neuronal firing rate was steady, the effects of agonists and/or antagonist ligands given alone or in combination were tested. In all experiments possible current artefacts were minimized using the automatic current balancing available on the Neurophore system. In some experiments recording sites were marked by ionophoretic ejection of pontamine sky blue dye. At the end of the experiment the brainstem was removed and fixed in 10% formal saline. Frozen sections (50 μm) were cut and the location of the recording sites visualized and mapped onto standard sections of a rat brainstem (Paxinos & Watson, 1998).
Analysis of data
Arterial blood pressure, tracheal pressure, ECG, phrenic nerve activity and neuronal activity were recorded on videotape via a digital interface (Instrutech, VR100A, Digitimer Ltd) and on a PC hard disk accessed via an A/D interface (Cambridge Electronic Design (CED) 1401plus). An on-line derivation of heart rate was made from the ECG using a custom-written script for commercially available software (Spike 2, CED, Cambridge). Off-line analysis of recorded data was made using Spike 2 software (CED). Single unit activity was discriminated using a Spike Processor (D130, Digitimer Ltd) and displayed as a rate histogram. To investigate the effects of ligands on ongoing NTS neuronal activity, baseline and ligand-evoked neuronal firing rates (averaged over a 10–20 s period) were measured and compared. Peri-stimulus time histograms (PSTHs, 20 stimuli) were constructed to investigate the effect of ligands on the vagal-evoked response of NTS neurones. The total number of evoked spikes before and during ionophoretic application of the ligands were compared. The response of NTS neurones to cardiopulmonary afferent stimulation by PBG injected into the right atrium was analysed by counting the total number of spikes evoked from the beginning of the excitatory burst until activity returned to the pre-PBG level. Ligands were classed as evoking excitation or inhibition if activity, respectively, increased or decreased by more than 20% (Wang et al. 1996, 1997, 1998; Sévoz-Couche et al. 2000). All data are presented as mean ± s.e.m. except where indicated. Comparisons of the means were made with the Student's paired t test.
Drugs and solutions
The following drugs were freshly dissolved in 0.9% saline and their pH adjusted by addition of drops of either 0.1 m HCl or 0.1 m NaOH. dl-Homocysteic acid (100 mm; pH 8.5), phenylbiguanide (10 mm, pH 10); AMPA (20 mm; pH 8.5); N-methyl-d-aspartate (NMDA, 20 mm, pH 8.5); (±)-2-amino-5-phosphonopentanoic acid (AP-5, 20 mm, pH 8.5) (from Sigma-Alrdich, Poole, Dorset), granisetron (10 mm, pH 4; a gift from Glaxo Smithkline, Harlow). 6,7-Dinitroquinoxaline-2,4-dione (DNQX, 2.5 mm, pH 8.5) was dissolved in 2.5% DMSO. Pontamine sky blue dye (20 mg ml−1; from BDH, Poole, Dorset) was dissolved in 0.5 m sodium acetate.
Results
Identification of neurones
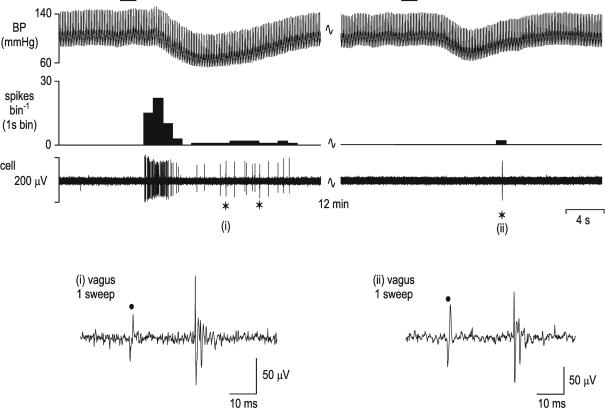
The NTS neurones studied in this investigation were identified by their response to electrical stimulation of the cervical vagus nerve (50–500 μA, 1 ms, 0.3–1 Hz). In total, 328 NTS neurones received an excitatory orthodromic input following stimulation of the vagus nerve. Sixty-one neurones received a vagal afferent input with a short (< 9 ms) minimum onset latency (range 3–9 ms, mean 5.9 ± 0.18 ms). This group will receive input from myelinated vagal afferents since their calculated conduction velocities (4.4–13.3 m s−1; mean 7.2 ± 0.26 m s−1) exclude non-myelinated fibres. However, the vast majority of neurones studied (n = 301) received a vagal afferent input with a much longer (> 20 ms) minimum onset latency (range 20–50 ms, mean 31.4 ± 0.33 ms). Thirty-four neurones received both short and longer latency inputs. One hundred and thirty three of those neurones receiving an excitatory input also showed a post-stimulation period of inhibition (duration range 80–750 ms, mean 325 ± 15 ms). In addition, another 10 neurones showed only a post-stimulus inhibition without any obvious excitatory response. The response of 200 of these NTS neurones to activation of cardiopulmonary receptors by PBG applied to the right atrium was tested. Such stimulation of cardiopulmonary afferents produced a burst of activity (41 ± 6 spikes) with mean latency to onset of 3.1 ± 0.3 ms in 128 neurones (Fig. 1). In another 33 neurones a period of inhibition (8.5 ± 1.8 s; latency 2.6 ± 0.2 ms) was seen whilst 12 neurones showed a biphasic response. The remaining 27 neurones were unaffected by cardiopulmonary afferent stimulation.
Figure 1. Identification of nucleus tractus solitarius neurones receiving vagal cardiopulmonary receptor inputs.
The response of a single nucleus tractus solitarius (NTS) neurone to activation of cardiopulmonary afferents by intra-atrial administration of phenylbiguanide (PBG, 12 μg kg−1, 20 μl) at the horizontal bar is shown in the left panel. This response is mediated by vagal afferents as the response to the same stimulus is abolished following bilateral cervical vagotomy (right panels). The neuronal recording is still intact following vagotomy since electrical stimulation of the cervical vagus central to the vagotomy (⋆) still evokes activity in the neurone (right panel). Top panels – from top, traces show arterial blood pressure (BP; mmHg), a continuous rate histogram of neuronal activity (spikes bin−1) and the raw recording of neuronal activity (μV). Bottom panels, single sweeps of neuronal activity evoked by electrical stimulation of the vagus nerve (•) before (i) and following (ii) vagotomy distal to the stimulating electrode at the points marked ⋆ in the panels above.
Effects of 5-HT3 receptor ligands on baseline neuronal activity
In 96 of 106 NTS neurones ionophoretic application of PBG (3–320 nA) significantly (P < 0.01) increased baseline firing rate from 2.4 ± 0.4 to 5.5 ± 0.8 spikes s−1 and in 92 of these neurones the recording continued post-PBG and cell activity recovered to 2.5 ± 0.5 spikes s−1. In another 9 neurones PBG was without significant effect on firing rate (3.7 ± 1.0 versus 4.0 ± 1.1 spikes s−1). Activity was decreased in only 1 of these 106 neurones. The vast majority (97) of these 106 neurones received a long latency vagal afferent input. Nine of these also received a short latency input, with another 9 neurones receiving input solely at short latency. Seven of these 9 neurones are included within the 96 neurones excited by ionophoretic application of PBG, the other 2 neurones being unaffected. From the population of 96 neurones whose activity was increased by ionophoretic application of PBG, the response to right atrial PBG (cardiopulmonary afferent input) was investigated only in 46 of those neurones that received long latency (since this stimulus will activate non-myelinated vagal afferent fibres). Forty-two neurones had such input. Thirty neurones were excited by this stimulus, 9 showed a pure inhibitory response and 3 a biphasic excitatory/inhibitory response. Only 4 neurones were unaffected by this stimulus. This range of responses is very similar in proportion to that seen in the entire population of 200 neurones described above.
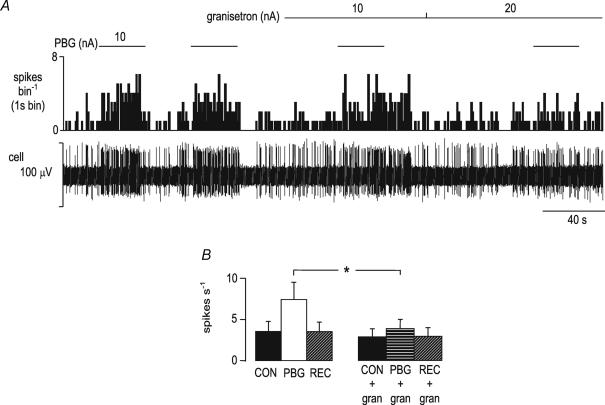
This increase in neuronal activity evoked by PBG (3.4 ± 1.1 to 7.0 ± 1.9 spikes s−1) was significantly (P < 0.05) attenuated by ionophoretic application of granisetron (5–60 nA) (3.0 ± 0.9 to 3.8 ± 1.1 spikes s−1; Fig. 2A) in all 17 neurones tested. In 15 of these neurones full recovery was recorded following PBG application (Fig. 2B). In the majority of neurones (11 of 18) granisetron was without effect on baseline firing rate (3.5 ± 1.2 to 3.6 ± 1.4 spikes s−1). In 4 neurones granisetron evoked a small but significant (P < 0.05) increase in activity (2.0 ± 0.7 to 2.5 ± 0.8 spikes s−1) whilst in the remaining 3 neurones activity was decreased during granisetron administration (5.5 ± 4.4 to 3.0 ± 2.4 spikes s−1) though this did not reach statistical significance. The effect of granisetron on the excitatory response to the excitant amino acid dl-homocysteic acid (DLH) was tested in 5 neurones in which granisetron had no effect on baseline activity. In 4 neurones the excitation (0.8 ± 0.3 to 3.3 ± 1.1 spikes s−1) was unaltered by application of granisetron (1.0 ± 0.3 to 3.5 ± 1.2 spikes s−1). In only one neurone was the response attenuated (by 46%) but in this neurone the excitation evoked by ionophoretic application of PBG was attenuated by 76%.
Figure 2. The excitatory effect of ionophoretic administration of a selective 5-HT3 receptor agonist (phenylbiguanide, PBG) is attenuated by the selective 5-HT3 receptor antagonist granisetron.
A, the excitatory response of an NTS neurone to the ionophoretic administration of phenylbiguanide (PBG, 10 nA) during the bar is attenuated when given in the presence of ionophoretic administration of granisetron (20 nA). From the top, traces show a continuous rate histogram (spikes bin−1) and the raw extracellular recording of neuronal activity (cell, μV). B, histograms of the mean data of NTS neuronal activity with vertical bars representing s.e.m. From left: baseline activity (CON), activity evoked by ionophoretic administration of PBG alone (5–120 nA; PBG), or in the presence of granisetron (5–60 nA; PBG + gran), and recovery of baseline activity (REC). *P < 0.05, n = 15.
Effects of 5-HT3 receptor ligands on vagal afferent-evoked responses and cardiopulmonary responses
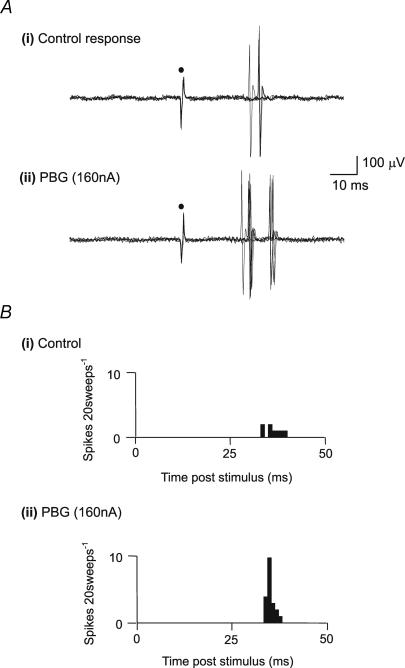
In 13 of 17 NTS neurones tested the excitatory response evoked by electrical stimulation of the cervical vagus nerve was significantly (P < 0.01) potentiated by ionophoretic application of PBG (11.5 ± 3.7 to 19.8 ± 3.9 spikes (20 sweeps)−1; Fig. 3). In 11 of these 13 neurones recordings were maintained following application of PBG, and activity was found to return to control levels (12.6 ± 4.3 before, 20.4 ± 4.6 during, 14.2 ± 4.9 spikes (20 sweeps)−1 after). Vagal-evoked activity was unaffected by PBG application in the other 4 neurones tested. Fifteen of these neurones received a long latency vagal afferent input with only 2 receiving a short latency input. These 2 neurones were among the group of 13 neurones whose input was potentiated by ionophoretic application of PBG. Seven of those cells whose input was potentiated by PBG were investigated for cardiopulmonary afferent input – six neurones were excited by right atrial PBG whilst the seventh showed a biphasic inhibition/excitation response.
Figure 3. Effect of 5-HT3 receptor ligands on vagal afferent-evoked activation of NTS neurones.
A, response of an NTS neurone to electrical stimulation of the vagus nerve at •. The traces show 5 superimposed sweeps of extracellular recorded neuronal activity evoked at a latency typical of nonmyelinated afferents, before (i) control, and during (ii) ionophoretic administration of PBG. B, post-stimulus time histograms (20 sweeps, 1 ms bins) of vagal afferent-evoked activity of an NTS neurone showing (i) control evoked activity (ii) and potentiation of this activity during the ionophoretic administration of PBG.
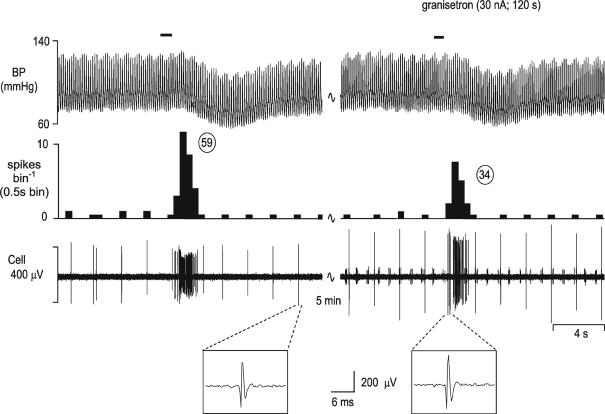
In addition, PBG application increased the response of cardiopulmonary afferent activation from 2.5 to 6.5 spikes burst−1 in 2 of the 3 neurones tested but was without effect on the third neurone. More importantly from a functional viewpoint, application of the 5-HT3 receptor antagonist granisetron significantly (P < 0.01) attenuated the cardiopulmonary afferent-evoked input (20.2 ± 5.7 to 10.6 ± 4.1 spikes burst−1) in 9 of 10 neurones tested (Fig. 4).
Figure 4. Attenuation of the cardiopulmonary afferent-evoked activation of NTS neurones by ionophoretic administration of granisetron.
Activation of cardiopulmonary afferents by intra-atrial administration of phenylbiguanide (PBG, 24 μg kg−1, 40 μl) at the solid horizontal line evokes a burst of activity (59 spikes) in an NTS neurone (left panel). This excitatory response is reduced to 34 spikes when the same stimulus is give during ionophoretic application of granisetron (30 nA; right panel). From top, traces show arterial blood pressure (BP; mmHg), a continuous rate histogram of neuronal activity (spikes bin−1) and the raw recording of neuronal activity (μV). Expanded panels below confirm the identity of the cell between panels.
Effects of glutamate receptor ligands on NTS neuronal activity
Ionophoretic application of NMDA (20–240 nA, n = 51) and AMPA (5–120 nA; n = 44) significantly (P < 0.01) increased activity of all neurones tested (NMDA, 0.8 ± 0.2 to 4.8 ± 0.5 spikes s−1; AMPA, 1.2 ± 0.3 to 7.1 ± 1.6 spikes s−1; Figs 5, 6 and 7). In the 13 neurones tested, the AMPA-evoked increase in activity (0.6 ± 0.3 to 8.2 ± 0.8 spikes s−1) was significantly (P < 0.01) attenuated (0.3 ± 0.1 to 2.6 ± 0.7 spikes s−1) by the non-NMDA receptor antagonist DNQX (10–80 nA; Fig. 5). At the ionophoretic currents applied these effects were selective for non-NMDA receptors since the NMDA-evoked excitation of these neurones (0.5 ± 0.2 to 6.4 ± 0.9 spikes s−1) was unchanged by application of DNQX (0.4 ± 0.1 to 5.9 ± 1.1 spikes s−1; Fig. 5). Similarly, in 15 of the 18 neurones tested the significant (P < 0.01) increase in activity (0.8 ± 0.3 to 6.1 ± 1.1 spikes s−1) evoked by NMDA was significantly (P < 0.01) attenuated (0.8 ± 0.3 to 3.2 ± 0.9 spikes s−1) by the NMDA receptor antagonist AP-5 (10–120 nA; Figs 6 and 7) and again, at these ionophoretic currents the effects were selective for NMDA receptors since AP-5 had no effect on the increase in activity evoked by AMPA (0.6 ± 0.3 to 4.7 ± 1.6 spikes s−1 before AP-5; 0.7 ± 0.3 to 5.0 ± 2.0 spikes s−1 during AP-5) in 6 of 12 neurones (Fig. 6). Interestingly, in 5 neurones, AP-5 significantly (P < 0.01) potentiated the excitatory response (0.8 ± 0.4 to 5.9 ± 1.9 spikes s−1 before to 1.2 ± 0.3 to 9.8 ± 2.3 spikes s−1 after; Fig. 7).
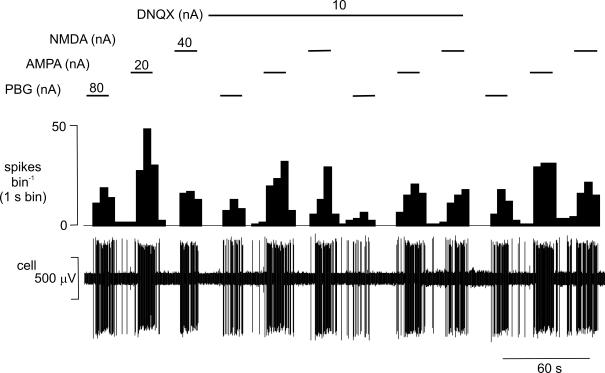
Figure 5. The effect of ionophoretic application of DNQX on the excitatory responses evoked by PBG, NMDA and AMPA ionophoretically applied to an NTS neurone.
Ionophoretic administration of PBG, AMPA and NMDA (during the respective bars and at the currents stated) all evoked excitatory responses in an NTS neurone. The responses to PBG and AMPA, but not those to NMDA are attenuated during ionophoretic administration of the selective non-NMDA receptor antagonist DNQX. From the top, traces show a continuous rate histogram (spikes bin−1) and the raw extracellular recording of neuronal activity (cell, μV).
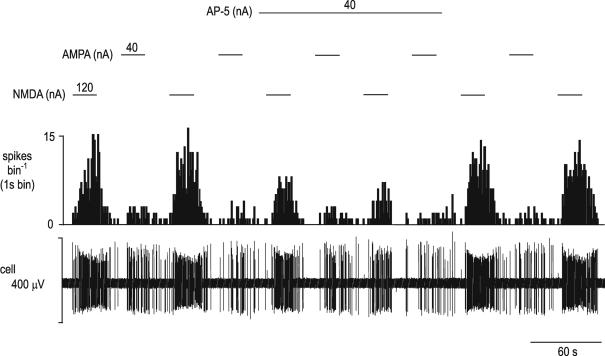
Figure 6. The effect of ionophoretic application of AP-5 on the excitatory responses evoked by NMDA and AMPA ionophoretically applied to an NTS neurone.
Ionophoretic administration of AMPA and NMDA (during the respective bars and at the currents stated) evoke excitatory responses in an NTS neurone. The responses to NMDA but not those to AMPA are attenuated during ionophoretic administration of the selective NMDA receptor antagonist AP-5. From the top, traces show a continuous rate histogram (spikes bin−1) and the raw extracellular recording of neuronal activity (cell, μV).
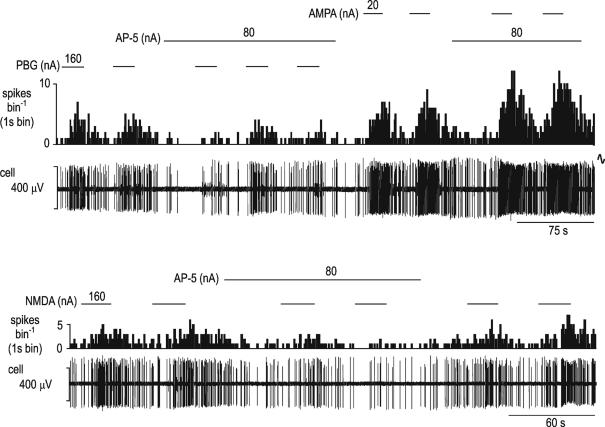
Figure 7. The effect of ionophoretic application of AP-5 on the excitatory responses evoked by phenylbiguanide, NMDA and AMPA ionophoretically applied to an NTS neurone.
Ionophoretic administration of PBG, AMPA and NMDA (during the respective bars and at the currents stated) all evoke excitatory responses in an NTS neurone. The responses to PBG (top panels) and NMDA (bottom panels), but not those to AMPA (top panels) are attenuated during ionophoretic administration of the selective NMDA receptor antagonist AP-5. The two panels are separated by about 60 s. In each panel traces show a continuous rate histogram (spikes bin−1) and raw extracellular recording of neuronal activity (cell, μV).
Effects of glutamate receptor ligands on response of NTS neurones to 5-HT3 receptor ligands
Ionophoretic application of DNQX (30–80 nA) significantly (P < 0.01) attenuated the increase in NTS neuronal activity evoked by ionophoretic application of PBG (Fig. 5) in all 8 neurones tested (2.9 ± 1.2 to 3.9 ± 1.3 spikes s−1 before DNQX; 2.5 ± 1.0 to 2.5 ± 1.0 spikes s−1 during DNQX). In addition it significantly (P < 0.01) attenuated both vagal-evoked excitation (35.8 ± 7.5 to 15.3 ± 1.9 spikes (20 sweeps)−1) in all 12 neurones tested and cardiopulmonary afferent-evoked excitation (56.9 ± 24.6 to 32.6 ± 19.7 spikes burst−1) in 5 of the 8 neurones tested (Fig. 8). Similarly, AP-5 (40–120 nA) significantly (P < 0.01) attenuated both the excitation of NTS neurones by ionophoretically applied PBG (1.2 ± 0.9 to 4.4 ± 1.8 spikes s−1 before AP-5; 1.1 ± 0.7 to 1.9 ± 0.8 spikes s−1 during AP-5) in 5 of 7 neurones tested (Fig. 7) and the cardiopulmonary afferent-evoked excitation (45 ± 12 to 19 ± 4 spikes burst−1) in 3 of 5 neurones tested (Fig. 9).
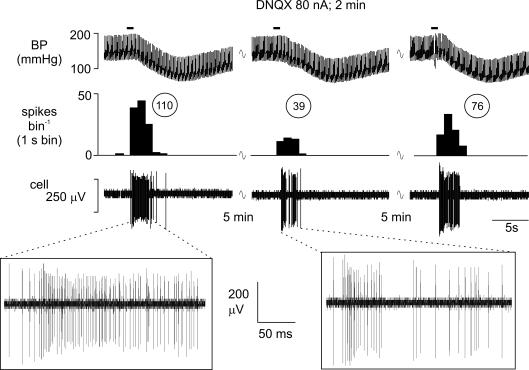
Figure 8. Attenuation of the cardiopulmonary afferent-evoked activation of an NTS neurone by ionophoretically applied DNQX.
Activation of cardiopulmonary afferents by intra-atrial administration of phenylbiguanide (PBG, 12 μg kg−1, 20 μl) at the solid horizontal line evokes a burst of activity (110 spikes) in an NTS neurone (left panel). This excitatory response is reduced to 39 spikes when the same stimulus is given during ionophoretic application of the selective non-NMDA receptor antagonist DNQX (80 nA; middle panel) and recovers to 76 spikes 5 min after removal of the DNQX (right panel). From the top, traces show arterial blood pressure (BP; mmHg), a continuous rate histogram of neuronal activity (spikes bin−1) and the raw recording of neuronal activity (cell, μV).
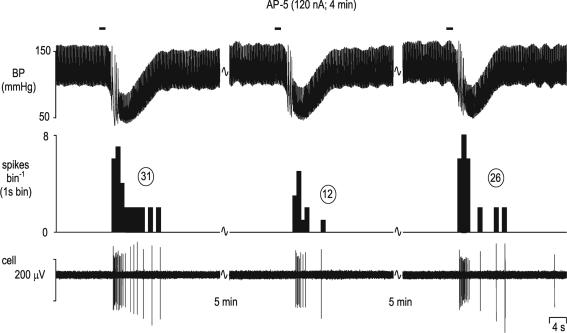
Figure 9. Attenuation of the cardiopulmonary afferent-evoked activation of an NTS neurone by ionophoretically applied AP-5.
Activation of cardiopulmonary afferents by intra-atrial administration of phenylbiguanide (PBG, 12 μg kg−1, 20 μl) at the solid horizontal lines evokes a burst of activity (31 spikes) in an NTS neurone (left panel). This excitatory response is reduced to 12 spikes when the same stimulus is given during ionophoretic application of the selective NMDA receptor antagonist AP-5 (120 nA; middle panel) and recovers to 26 spikes 5 min after removal of the AP-5 (right panel). From the top, traces show arterial blood pressure (BP; mmHg), a continuous rate histogram of neuronal activity (spikes bin−1) and the raw recording of neuronal activity (cell, μV).
Discussion
The present experiments have demonstrated in anaesthetized rats that activity of NTS neurones receiving inputs from vagal afferents can be increased by ionophoretic application of the 5-HT3 receptor agonist phenylbiguanide (PBG). This excitation included, but was not necessarily exclusive to those cells responsive to stimulation of non-myelinated cardiopulmonary afferents. Further, in the presence of the selective 5-HT3 receptor antagonist granisetron (Sanger & Nelson, 1989), applied by ionophoresis, this PBG-evoked excitation was significantly attenuated whilst excitation evoked by the excitant amino acid dl-homocysteic acid was unaffected. This confirms that the PBG-evoked excitation is mediated by 5-HT3 receptors and is not due to a non-specifc action of this agonist. As the NTS has the highest density of brainstem 5-HT3 receptors in both rat (Laporte et al. 1992; Steward et al. 1993; Morales et al. 1998; Miquel et al. 2002) and human tissues (Parker et al. 1996) this would indicate that the predominant effect of activation of these 5-HT3 receptors is one of excitation. Whilst some of these receptors are located on postsynaptic sites, many are located on vagal afferent terminals (presynaptically) since vagotomy substantially reduced 5-HT3 binding (Pratt & Bowery, 1989; Kidd et al. 1993; Leslie et al. 1994). This would also seem to apply to glossopharyngeal afferents which mediate baroreceptor and chemoreceptor reflexes since many petrosal ganglion cells express 5-HT3 receptor mRNA (Wang et al. 2002). In addition, a recent ultrastructural study of the medial NTS has demonstrated the presence of immunoreactivity for the 5-HT3A receptor subunit on small non-myelinated axons and axon terminals, on somatodendritic and glial profiles (Huang et al. 2004). These data would be consistent with in vitro electrophysiological studies showing that activating 5-HT3 receptors produced depolarization and/or increased spontaneous postsynaptic potentials in NTS neurones (Glaum et al. 1992).
Interestingly, at least in this anaesthetized rat preparation, these 5-HT3 receptors do not seem to be tonically activated since application of the selective antagonist granisetron alone rarely affected the ongoing firing rate of NTS neurones. This would support previous data from NTS microinjections of 5-HT3 receptor antagonists which were also without effect on baseline blood pressure and heart rate in both anaesthetized (Sévoz et al. 1996; Pires et al. 1998) and awake rats (Callera et al. 1997), though in a similar study Ramage & Mifflin (1998) reported that granisetron did significantly reduce ongoing NTS activity. The present data suggest that NTS neurones are activated by 5-HT3 receptors only when reflex inputs are stimulated. In this respect, ionophoresis of PBG potentiated vagal input to these NTS neurones, whether by electrical stimulation of the vagus nerve or by chemical activation of cardiopulmonary afferent fibres. The neurones investigated in the present study could be split into two groups based on the onset latency of their response to electrical stimulation of the vagus nerve. One group was activated at short latency (< 9 ms) and it is likely that they receive input from myelinated vagal afferent fibres since their calculated conduction velocities are too fast to include non-myelinated fibres. The majority of neurones studied had vagal inputs with much longer (> 20 ms) onset latencies. Many of these, including those activated by cardiopulmonary afferents, will receive non-myelinated vagal afferent input. Indeed, when a population of 46 of those neurones receiving long latency vagal input were tested, 91% (42 neurones) were shown to receive a functional non-myelinated afferent input from cardiopulmonary afferent stimulation. However, we cannot exclude that this group also receives input from faster conducting afferent fibres mediated by polysynaptic pathways. The present data demonstrate that these synaptic inputs were attenuated by application of granisetron. This is consistent with previous reports in the literature that antagonism of 5-HT3 receptors with granisetron can reduce reflex responses to upper airway (Dando et al. 1995) and cardiopulmonary afferents (Pires et al. 1998) and vagal afferent-evoked excitation of NTS neurones (Ramage & Mifflin, 1998). In contrast, there are other reports that show that exogenously activating 5-HT3 receptors can reduce the reflex falls in heart rate evoked by stimulating baroreceptor (Sévoz et al. 1996; Callera et al. 1997; Bonagamba et al. 2000), chemoreceptor (Callera et al. 1997; Sévoz et al. 1997) and cardiopulmonary afferents (Sévoz et al. 1996; Leal et al. 2001). However, activating 5-HT3 receptors generally has an excitatory effect on neuronal activity, as indeed we have demonstrated in the present study – baseline activity and synaptic transmission of vagal inputs was increased when 5-HT3 receptors were activated, and attenuated when these receptors were antagonized. Thus, these authors argued that their opposite data could be explained if the release of glutamate activated an inhibitory interneurone since GABAA receptor antagonists attenuated the 5-HT3 receptor-mediated modulation of the reflex responses (Sévoz et al. 1996, 1997). If this were the case, then in sampling a large population of NTS neurones one might expect to see some neurones excited by activation of 5-HT3 receptors (the inhibitory interneurones) and another population which were inhibited (downstream of the interneurone). However, in our sample of 97 neurones whose activity was affected by ionophoretic application of PBG the vast majority (99%) were excited and only a single neurone was inhibited. It is extremely unlikely that our ‘random’ sampling recorded all inhibitory interneurones. The present data also would be consistent with the effect on the reflex responses reported by Pires et al. (1998) who demonstrated that following i.c. application or NTS microinjection, granisetron dose dependently attenuated the bradycardia evoked by activating cardiopulmonary receptors. Activation of 5-HT3 receptors has been reported similarly to modulate synaptic transmission in other sensory nuclei such as the dorsal horn of the spinal cord where they modulate nociceptive responses (Glaum et al. 1990; Sasaki et al. 2001) and the reflex responses to bladder afferent stimulation (Espey et al. 1998).
The present data demonstrating that NTS cells can be activated by both NMDA and non-NMDA receptor ligands is confirmation of evidence in the literature that both vagal primary afferent fibres and intrinsic NTS neurones use glutamate as an excitatory neurotransmitter (Saha et al. 1995; Zhang & Mifflin, 1997; Jones et al. 2002). Importantly however, both DNQX, a selective non-NMDA receptor antagonist (Honoréet al. 1988), and AP-5, a selective NMDA receptor antagonist (Lodge et al. 1988), attenuated not only cardiopulmonary-evoked excitation of NTS neurones but also that produced by ionophoretic application of PBG. Since not all cardiopulmonary inputs were blocked by these antagonists it might be concluded that some were mediated by transmitters other than glutamate. However, one should not rule out the possibility that even in these neurones cardiopulmonary inputs are indeed glutamatergic. Using ionophoresis it is often more difficult to antagonize synaptic as opposed to exogenously applied agonists so negative data cannot always be relied upon. Extracellular recordings cannot rule out some direct postsynaptic facilitatory action of 5-HT3 receptors on NTS neurones. Additionally, however, the present data, taken in conjunction with the known presynaptic location of many NTS 5-HT3 receptors, would be consistent with the hypothesis that part of the activation of NTS neurones produced by 5-HT3 receptors may be indirect and mediated via a glutamatergic mechanism. Indeed, Ashworth-Preece et al. (1995) have previously shown by microdialysis that PBG can stimulate rises in extracellular levels of glutamate in the NTS and most recently, Reges et al. (2002) have shown in awake rats that microinjection of AP-5 into NTS blocks the pressor response to microinjecting 2-methyl 5-HT (which will activate 5-HT3 receptors). This dependence of 5-HT3 receptor responses on the integrity of glutamatergic transmission is not unlike that reported as operating within the dorsal vagal nucleus of the rat (Wang et al. 1996, 1998) and again is consistent with the view that activating 5-HT3 receptors, located on vagal afferent fibres, were facilitating release of glutamate from the afferent fibres. A similar mechanism could account for the data in the present report. However, the data would be consistent with another exciting possibility – that 5-HT3 receptors located on glia activate these cells to release glutamate which would then act on neuronal glutamate receptors. This possibility arises since, despite the wealth of functional data (see above), a recent light microscope and electron microscope study reported that barosensitive neurones within the NTS received few, if any, direct appositions from 5-HT-containing terminals. More usually, thin glial processes were interposed between serotonergic terminals and NTS cells bodies (Llewellyn-Smith et al. 2004). Additionally, Huang et al. (2004) demonstrated 5-HT3A receptor subunit immunoreactivity on glial profiles within the medial NTS. This is intriguing since glial cells are known to express receptors for a range of neurotransmitters, including 5-HT (Porter & McCarthy, 1997) and their activation evokes an increase in [Ca2+]i (Hagberg et al. 1998) and subsequent release of glutamate (Meller et al. 2002) which can modify synaptic activity (see Fellin & Carmignoto, 2004). Thus, some of the functional effects of 5-HT3 receptor activation may involve glial cells as intermediaries, rather than direct actions on NTS neurones.
In conclusion, the present study has demonstrated in anaesthetized rats in vivo that synaptic transmission of vagal afferent inputs within the NTS, including those from non-myelinated cardiopulmonary afferents, can be increased by activating 5-HT3 receptors. The attenuation of these inputs by a selective 5-HT3 receptor antagonist granisetron implies that these inputs release 5-HT within the NTS. This 5-HT3 receptor-mediated facilitation of transmission may in part be a direct action on postsynaptic 5-HT3 receptors but in part also appears to involve release of glutamate since selective antagonism of either non-NMDA or NMDA receptors attenuated the 5-HT3 receptor-mediated facilitations. The origin of this glutamate remains to be resolved.
Acknowledgments
R.D.J. and D.O.K. were supported by British Heart Foundation PhD Studentships and Y.W. was supported by the Wellcome Trust (Grant 050894/Z). We are grateful for the technical assistance of Mr S Wilkinson. Granisetron was a generous gift from GSK Harlow UK.
References
- Ashworth-Preece MA, Jarrot B, Lawrence AJ. 5-Hydroxytryptamine3 receptor modulation of excitatory amino acid release in the rat nucleus tractus solitarius. Neurosci Lett. 1995;191:75–78. doi: 10.1016/0304-3940(95)11564-5. [DOI] [PubMed] [Google Scholar]
- Bogle RG, Pires JG, Ramage AG. Evidence that central 5-HT1A receptors play a role in the von Bezold-Jarisch reflex in the rat. Br J Pharmacol. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonagamba LG, Sévoz-Couche C, N'Diaye A, Uygun-Louvet K, Callera J, Machado BH, et al. Bradycardic responses to microinjection of N-methyl-D-aspartate into the nucleus tractus solitarius are inhibited by local activation of 5-HT3 receptors. Neurophamacology. 2000;39:2336–2345. doi: 10.1016/s0028-3908(00)00093-9. [DOI] [PubMed] [Google Scholar]
- Brooks PA, Albert A. 5-HT3 receptors in the dorsal vagal complex. In: Reynolds DJM, Andrews PLR, Davis CJ, editors. Serotonin and the Scientific Basis of Anti-Emetic Therapy. Oxford: Oxford Clinical Communications; 1995. pp. 106–110. [Google Scholar]
- Callera JC, Sévoz C, Laguzzi R, Machado BH. Microinjection of a serotonin3 receptor agonist into the NTS of unanesthetized rats inhibits the bradycardia evoked by activation of the baro- and chemoreflexes. J Autonom Nerv Syst. 1997;63:127–136. doi: 10.1016/s0165-1838(96)00140-3. [DOI] [PubMed] [Google Scholar]
- Dando SB, Jordan D, Ramage AG. The role of central 5-HT3 receptors in the modulation of the response to upper airway stimulation in the anaesthetized rabbit. J Physiol. 1995;489:156–157P. [Google Scholar]
- Espey MJ, Du H-J, Downie JW. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Res. 1998;798:101–108. doi: 10.1016/s0006-8993(98)00401-6. [DOI] [PubMed] [Google Scholar]
- Feldman PD. Effects of serotonin-1 and serotonin-2 receptor agonists on neuronal activity in the nucleus tractus solitarius. J Autonom Nerv Syst. 1995;56:119–124. doi: 10.1016/0165-1838(95)00054-3. [DOI] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Brooks PA, Spyer KM, Miller RJ. 5-hydroxytryptamine-3 receptors modulate synaptic activity in the rat nucleus tractus solitarius in vitro. Brain Res. 1992;589:62–68. doi: 10.1016/0006-8993(92)91162-8. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Proudfit HK, Anderson EG. 5-HT3 receptors modulate spinal nociceptive reflexes. Brain Res. 1990;510:12–16. doi: 10.1016/0006-8993(90)90721-m. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br J Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg G-B, Blomstrand F, Nilsson M, Tamir H, Hansson E. Stimulation of 5-HT2A receptors on astrocytes in primary culture opens voltage-independent Ca2+ channels. Neurochem Int. 1998;32:153–162. doi: 10.1016/s0197-0186(97)00087-9. [DOI] [PubMed] [Google Scholar]
- Hines T, Toney GM, Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circ Res. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Honoré T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, et al. Quinoxalinediones: Potent competitive non-NMDA glutamate receptor anatgonists. Science. 1988;241:701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Huang J, Spier AD, Pickel VM. 5-HT3A receptor subunits in the rat medial nucleus of the solitary tract: subcellular distribution and relation to the serotonin transported. Brain Res. 2004;1028:156–169. doi: 10.1016/j.brainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Itoh H, Bunag RD. Cardiovascular and sympathetic effects of injecting serotonin into the nucleus tractus solitarius in rats. J Pharmacol Exp Ther. 1991;256:1147–1153. [PubMed] [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. The role of 5-HT3 receptors in the cardiopulmonary afferent-evoked response of NTS neurones in the anaesthetized rat. J Physiol. 2000;523P:258–259P. [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. Evidence to suggest that presynaptic 5-HT3 receptors are involved in the excitation of NTS neurones in anaesthetised rats. J Physiol. 2001;533P:92–93P. [Google Scholar]
- Jones GA, Llewellyn-Smith IJ, Jordan D. Physiological, pharmacological and immunohistochemical characterisation of juxtacellularly labelled neurones in rat nucleus tractus solitarius. Autonom Neurosci. 2002;98:12–16. doi: 10.1016/s1566-0702(02)00022-x. [DOI] [PubMed] [Google Scholar]
- Jordan D. Central nervous pathways and control of the airways. Resp Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: Central serotonergic (5-HT) mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Jordan D, Spyer KM. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- Kay IS, Armstrong DJ. Phenylbiguanide not phenyldiguanide is used to evoke the pulmonary chemoreflex in anaesthetized rabbits. Exp Physiol. 1990;75:383–389. doi: 10.1113/expphysiol.1990.sp003413. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Excitation of rat nucleus tractus solitarius (NTS) neurones by vagal afferents involves central 5-HT7 and AMPA receptors. J Physiol. 2004;560P:C10. [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Central 5-HT7 receptors are critical for reflex activation of cardiac vagal drive in anaesthetised rats. J Physiol. 2005;563:319–331. doi: 10.1113/jphysiol.2004.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd EJ, Laporte AM, Langlois X, Fattaccini CM, Doyen C, Lombard MC, et al. 5-HT3 receptors in the rat central nervous system are mainly located on nerve fibres and terminals. Brain Res. 1993;612:289–298. doi: 10.1016/0006-8993(93)91674-h. [DOI] [PubMed] [Google Scholar]
- Laguzzi R, Reis DJ, Talman W. Modulation of cardiovascular and electrocortical activity through serotonergic mechanisms in the nucleus tractus solitarius of the rat. Brain Res. 1984;304:321–328. doi: 10.1016/0006-8993(84)90336-6. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Koscielniak T, Ponchant M, Verge D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I]iodo-zacopride and [3H] zacopride as ligands. Synapse. 1992;10:271–281. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Leal DM, Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Microinjection of a 5-HT3 receptor agonist into the NTS of awake rats inhibits the bradycardic response to activation of the von Bezold-Jarisch reflex. Brain Res Bull. 2001;54:7–11. doi: 10.1016/s0361-9230(00)00408-1. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJM, Newberry NR. Localization of 5-HT3 receptors. In: King FD, Jones BJ, Sanger GJ, editors. 5-Hydroxytryptamine-3 Receptor Antagonists. Ann Arbor, MI, USA: CRC Press; 1994. pp. 79–96. [Google Scholar]
- Llewellyn-Smith IA, Kellett DO, Jones GA, Jordan D. Do serotonergic axons directly innervate cardiovascular neurons in rat nucleus tractus solitarius (NTS) ? J Physiol. 2004;557P:C99. [Google Scholar]
- Lodge D, Davies SN, Jones MG, Millar J, Manallack DT, Ornstein PL, et al. A comparison between the in vivo and in vitro activity of five potent and competitive NMDA antagonists. Br J Pharmacol. 1988;95:957–965. doi: 10.1111/j.1476-5381.1988.tb11726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- Meller R, Harrison PJ, Elliot JM, Sharp T. In vitro evidence that 5-hydroxytryptamine increases efflux of glial glutamate via 5-HT2A receptor activation. J Neurosci Res. 2002;67:399–405. doi: 10.1002/jnr.10126. [DOI] [PubMed] [Google Scholar]
- Merahi N, Orer HS, Laporte AM, Gozlan H, Hamon M, Laguzzi R. Baroreceptor reflex inhibition induced by the stimulation of serotonin3 receptors in the nucleus tractus solitarius of the rat. Neurosci. 1992;46:91–100. doi: 10.1016/0306-4522(92)90011-p. [DOI] [PubMed] [Google Scholar]
- Miquel M-C, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil M-J, et al. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5-HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- N'Diaye A, Sévoz-Couche C, Nosjean A, Hamon M, Laguzzi R. Stimulation of 5-HT2 receptors in the nucleus tractus solitarius enhances NMDA receptor-mediated reflex-evoked bradycardiac responses in the rat. Autonom Neurosci. 2001;92:45–55. doi: 10.1016/S1566-0702(01)00318-6. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJ. Localization of 5-ht5A receptor-like immunoreactivity in the rat brain. Brain Res. 2000;867:131–142. doi: 10.1016/s0006-8993(00)02273-3. [DOI] [PubMed] [Google Scholar]
- Parker RM, Barnes JM, Ge J, Barber PC, Barnes NM. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J Neurol Sci. 1996;144:119–127. doi: 10.1016/s0022-510x(96)00211-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. New York: Academic Press; 1998. [Google Scholar]
- Pires JG, Silva SR, Ramage AG, Futuro-Neto HA. Evidence that 5-HT3 receptors in the nucleus tractus solitarius and other brainstem areas modulate the vagal bradycardia evoked by activation of the von Bezold-Jarisch reflex in the anaesthetized rat. Brain Res. 1998;791:229–234. doi: 10.1016/s0006-8993(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG. The 5-HT3 receptor ligand [3H]BRL 43694 binds to presynaptic sites in the nucleus tractus solitarius of the rat. Neurophamacology. 1989;28:1367–1376. doi: 10.1016/0028-3908(89)90012-9. [DOI] [PubMed] [Google Scholar]
- Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Mifflin SW. Vagal-evoked excitation of a sub-population of neurones in the nucleus of the solitary tract (NTS) involves 5-HT3 receptors in the anaesthetized rat. J Physiol. 1998;509P:129P. [Google Scholar]
- Reges RV, Bonagamba LGH, Nosjean A, Laguzzi R, Machado BH. Involvement of NMDA receptors in the pressor response to microinjection of 5-HT3 agonist into the NTS of awake rats. Autonom Neurosci. 2002;98:2–6. doi: 10.1016/s1566-0702(02)00020-6. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TFC, McWilliam PN. Glutamate immunoreactivity in identified vagal afferent terminals of the cat: a study combining horseradish peroxidase tracing and electron microscopic immunogold staining. Exp Physiol. 1995;80:193–202. doi: 10.1113/expphysiol.1995.sp003839. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Nelson DR. Selective and functional 5-hydroxytryptamine3 receptor antagonism by BRL 43694 (granisetron) Eur J Pharmacol. 1989;159:113–124. doi: 10.1016/0014-2999(89)90695-x. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ishizaki K, Obata H, Gato F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur J Pharmacol. 2001;424:45–52. doi: 10.1016/s0014-2999(01)01117-7. [DOI] [PubMed] [Google Scholar]
- Sévoz C, Callera J-C, Machado B, Hamon M, Laguzzi R. Role of serotonin3 receptors in the nucleus tractus solitarii on the carotid chemoreflex. Am J Physiol. 1997;272:H1250–H1259. doi: 10.1152/ajpheart.1997.272.3.H1250. [DOI] [PubMed] [Google Scholar]
- Sévoz C, Nosjean A, Callera J-C, Machado B, Hamon M, Laguzzi R. Stimulation of 5-HT3 receptors in the NTS inhibits the cardiac Bezold-Jarisch reflex response. Am J Physiol. 1996;271:H80–H87. doi: 10.1152/ajpheart.1996.271.1.H80. [DOI] [PubMed] [Google Scholar]
- Sévoz-Couche C, Wang Y, Ramage AG, Spyer KM, Jordan D. In vivo modulation of nucleus tractus solitarius (NTS) neurones by activation of 5-hydroxytryptamine2 receptors in rats. Neurophamacology. 2000;39:2006–2016. doi: 10.1016/s0028-3908(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Distribution of serotonin immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steward LJ, West KE, Kilpatrick GJ, Barnes NM. Labelling of 5-HT3 receptor recognition sites in the rat brain using the agonist radioligand [3H]meta-chlorophenylbiguanide. Eur J Pharmacol. 1993;243:13–18. doi: 10.1016/0014-2999(93)90161-a. [DOI] [PubMed] [Google Scholar]
- Sykes RM, Spyer KM, Izzo PN. Central distribution of substance P, calcitonin gene-related peptide and 5-hydroxytryptamine in vagal sensory afferents in the rat dorsal medulla. Neuroscience. 1994;59:195–210. doi: 10.1016/0306-4522(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Taylor EW, Jordan D, Coote JH. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev. 1999;79:855–916. doi: 10.1152/physrev.1999.79.3.855. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. Mediation by 5-HT3 receptors of an excitatory effect of 5-HT on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. Br J Pharmacol. 1996;118:1697–1704. doi: 10.1111/j.1476-5381.1996.tb15594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. In vivo effects of 5-hydroxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neuropharmacology. 1997;36:489–498. doi: 10.1016/s0028-3908(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. Presynaptic 5-HT3 receptors evokes an excitatory response in dorsal vagal preganglionic neurones in anaesthetized rats. J Physiol. 1998;509:683–694. doi: 10.1111/j.1469-7793.1998.683bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-Y, Keith IM, Olson EB, Vidruk EH, Bisgard GE. Expression of 5-HT3 receptors in primary sensory neurons of the petrosal ganglion of adult rats. Autonom Neurosci. 2002;95:121–124. doi: 10.1016/s1566-0702(01)00384-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Influences of excitatory amino acid receptor agonists on nucleus of the solitary tract neurons receiving aortic depressor nerve inputs. J Pharmacol Exp Ther. 1997;282:639–647. [PubMed] [Google Scholar]