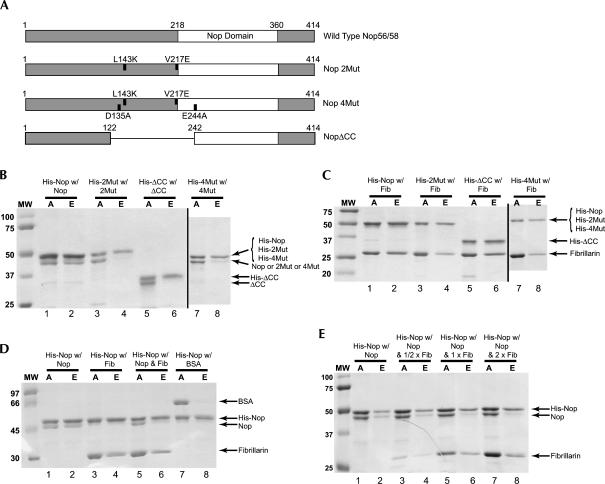
FIGURE 2.
Nop56/58 Self-dimerization and Nop56/58 dimerization with fibrillarin are mutually exclusive interactions. (A) Schematic presentation of the M. jannaschii Nop56/58 wild-type and mutant proteins. Mutated amino acids in the coiled-coil domain of the 2Mut and 4Mut Nop56/58 mutant proteins and the deleted domain of the ΔCC mutant protein are indicated. (B) Protein–protein pull-down analysis of wild-type and mutant Nop56/58 self-dimerization. Wild-type, 2Mut, 4Mut, or ΔCC Nop56/58 proteins were mixed with His-tagged protein and then self-dimerized protein pairs selected by nickel column chromatography. Total or applied protein (A) and His-tagged and nickel-selected or eluted (E) proteins were resolved by SDS gel electrophoresis and revealed by staining. Proteins used in individual incubations and selected in the eluted fractions are indicated above and at the side, respectively. (C) Mutation or deletion of the Nop56/58 coiled-coil domain does not affect dimerization with fibrillarin. Proteins used in the individual incubations (A) and observed in the selected fractions (E) are indicated above and at the side, respectively. (D) Nop56/58p self-dimerization and Nop56/58 dimerization with fibrillarin are mutually exclusive protein:protein interactions. Proteins used in the individual incubations (A) and observed in the selected fractions (E) are indicated above and at the side, respectively. (E) Titration of the Nop56/58 homodimer with increasing amounts of fibrillarin disrupts Nop56/58 self-dimerization. Constant amounts of His-tagged and untagged Nop56/58 were incubated with increasing concentrations of fibrillarin protein (lanes 3,5,7). Interacting proteins were selected by nickel chromatography of the His-tagged Nop56/58 and resolved by SDS gel electrophoresis (lanes 4,6,8).