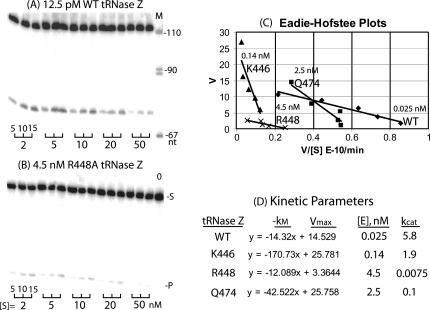
FIGURE 4.
Kinetics of processing with wild-type and variant tRNase Z. Mutations analyzed are the same as in Figure 2. (A,B) The processing in a kinetic experiment with wild-type and R448A tRNase Z. Reactions were sampled after 5, 10, and 15 min as designated by the brackets below the data panels. The concentrations of tRNase Z (12.5 pM for wild type and 4.5 nM for R448A, designated above the panels) were chosen to produce a similar extent of processing (% product/minute of reaction, equivalent to V/[S]). The substrate concentrations, adjusted by adding unlabeled fruit fly pre-tRNAHis, were 2, 5, 10, 20, and 50 nM, as indicated below the panels. (C) Eadie-Hofstee plots for wild type, K446, R448, and Q474. Concentrations of enzymes used are indicated on the plot. (D) Kinetic parameters from the data presented in A–C. Equations presented for the enzymes (columns labeled –kM, Vmax, with units of × 10−9 M and × 10−11 M/min., respectively) are taken from the Excel plots (C). kcat is calculated from Vmax using the value for [E] determined from the protein gel lane for each mutant (Fig. 1) based on the dilution factor used in the experiment.