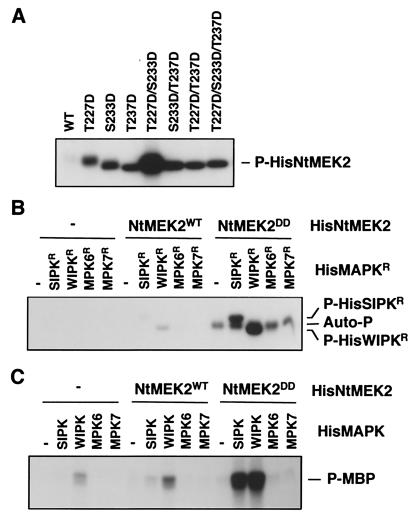
Figure 2.
Constitutively active NtMEK2 mutant phosphorylates and activates SIPK and WIPK. (A) NtMEK2DD, the T227D/S233D double mutant of NtMEK2, has elevated kinase activity. Autophosphorylation activities of 0.5 μg of His-tagged wild-type (WT) NtMEK2 and its mutants were determined as described in Materials and Methods. (B) NtMEK2DD preferentially phosphorylates SIPK and WIPK. Phosphorylation activities of HisNtMEK2WT and HisNtMEK2DD (0.1 μg) were determined by using inactive mutant MAPKs (HisMAPKR, 1 μg) as substrates. Reactions in the absence (−) of either MAPK or MAPKK were used as controls. (C) Phosphorylation by NtMEK2DD activates SIPK and WIPK. Wild-type MAPKs (HisMAPK, 1 μg) were incubated in the absence (−) or with 0.1 μg of HisNtMEK2WT or HisNtMEK2DD in the presence of 50 μM unlabeled ATP. MBP (final concentration of 0.25 μg/μl) and [γ-32P]ATP (1 μCi per reaction) were then added. Phosphorylated MBP (P-MBP) that reflects the activity of MAPK was visualized by autoradiography after SDS/PAGE.