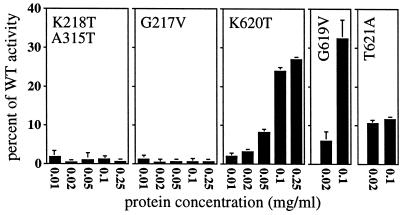
Figure 2.
Effects of protein concentration on the ATPase activity of mutant Hsp104 proteins. ATPase assays were performed with 5 mM ATP, using the Malachite Green colorimetric assay to measure released phosphate. The same buffer conditions were used as in cross-linking studies with different concentrations of Hsp104. The endpoints of reactions were varied to keep them in the linear range of the assay and subsequently adjusted to min−1. Mutant activity is shown as the % of WT activity in the same experiment; mean and standard deviations are shown. WT Hsp104 released 0.67, 0.77, 1.10, 0.89, 1.02, and 0.80 nmol of Pi min−1 μg−1, respectively, at 0.01, 0.02, 0.05, 0.1, 0.25, and 0.5 mg/ml. ATP hydrolysis by the K218T:A315T and G217V mutants was negligible at all protein concentrations, but the low hydrolysis by the K620T and G619V mutants increased more than 10-fold at high protein concentrations. No change occurred in the T621A mutant, which was oligomerized at all protein concentrations.