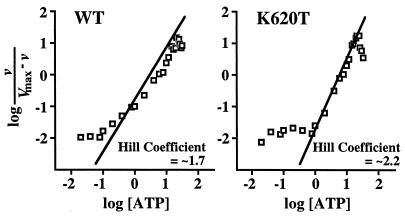
Figure 5.
Cooperativity of ATP hydrolysis. Kinetic analysis of K620T mutant and WT Hsp104 (0.25 mg/ml) ATP hydrolysis at ATP concentrations ranging from 20 μM to 32 mM. ATP hydrolysis was cooperative, yielding a Hill coefficient of ≈2.2 (standard error 0.2) for K620T and ≈1.7 (standard error 0.2) for WT. The rate of hydrolysis was calculated during the first 4 min of incubation with ATP for WT and 7 min for K620T to ensure that reaction products were measurable within the linear range of the assay. The Hill coefficients were calculated by fitting the nonlinear curves from plotting millimolar ATP vs. nmol of Pi released min−1 μg−1 to the Michaelis–Menten equation by using the allosteric kinetic equation of the GRAFIT 4 graphics program (Erithacus Software) and were similar to those calculated from the slope of the linear Hill plots shown.