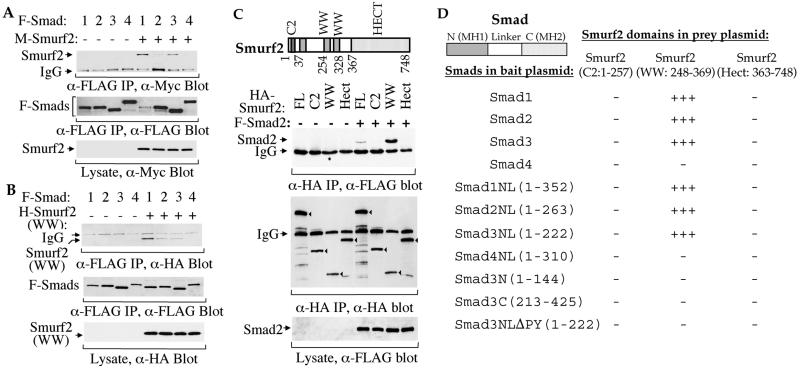
Figure 2.
Physical interactions of Smurf2 with Smads. (A) Full-length Smurf2 interacts with Smad1, Smad2, and Smad3 in mammalian cells. COS-1 cells were transfected with expression plasmids for FLAG (F)-tagged full-size Smad1, Smad2, Smad3, or Smad4 or Myc (M)-tagged Smurf2, as marked. Cell lysates were subjected to anti-FLAG immunoprecipitation followed by anti-Myc immunoblotting. (Top) Coprecipitation of M-Smurf2 with F-Smad1, Smad2, or Smad3 is shown. The levels of F-Smads in the immunoprecipitates (Middle) and M-Smurf2 in total cell lysates (Bottom) are shown as indicated. (B) WW domains of Smurf2 (amino acids 248–369) interact with Smad1, Smad2, or Smad3. Lysates of COS-1 cells, transfected with F-Smad1, Smad2, Smad3, or Smad4 and/or HA-tagged WW domains of Smurf2 expression plasmids, were subjected to anti-FLAG immunoprecipitation followed by anti-HA immunoblotting to detect association of HA-Smurf2 (WW) with F-Smad1, Smad2, or Smad3 (Top). The levels of F-Smads in the immunoprecipitates (Middle) and H-Smurf2 (WW) in the total cell lysates (Bottom) are shown as indicated. (C) WW domains (amino acids 248–369) but not C2 or Hect domains of Smurf2 interact with Smad. COS-1 cells were transfected with HA-tagged Smurf2 (FL), HA-Smurf2 fragments (C2, WW, or Hect) and/or F-Smad2, as indicated. Immunoprecipitation with anti-HA antibodies was followed by anti-FLAG immunostaining to detect Smurf2-associated Smad2 (top). (Middle) The anti-HA immunoprecipitated Smurf2 or its fragments (indicated by arrowheads) is shown. (Bottom) The expression level of Smad2 is shown. (D) Yeast two-hybrid assays demonstrate the interaction of Smad1, Smad2, and Smad3, but not Smad4, with the WW domain segment, and the requirement of the linker (L) region and the PPXY sequence of Smads. Interactions were scored by measuring the β-galactosidase activity from − (negative) to +++ (strongly positive).