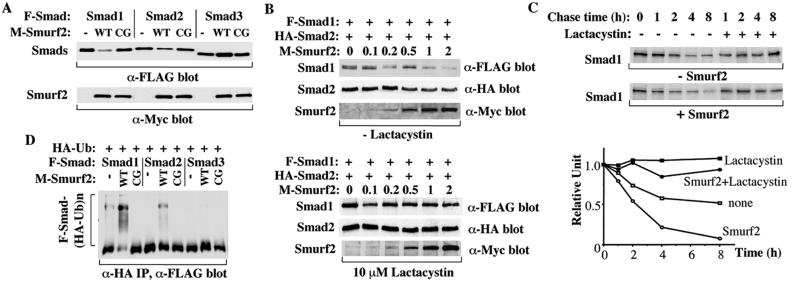
Figure 3.
Effect of Smurf2 on Smad1 and Smad2 levels. Smurf2 expression results in a dramatic decrease of Smad1 protein level and a slight decrease of Smad2 protein level. COS-1 (A, D) or 293 (B, C) cells were transfected with the indicated mammalian expression plasmids. (A) Smurf2, but not the catalytic inactive mutant of Smurf2 (CG), decreases the Smad1 steady-state levels dramatically and the Smad2 steady-state levels slightly. Smurf2 does not decrease the Smad3 levels. Steady-state protein levels were determined by immunoblotting aliquots of the total cell lysates. (B) The decrease of Smad1 and Smad2 protein levels depends on the expression levels of Smurf2 and can be inhibited by lactacystin. Cells transfected with F-Smad1, HA-Smad2, and increasing amounts of Myc-Smurf2 expression plasmid were treated overnight without (Upper) or with (Lower) lactacystin before lysis of cells and immunostaining for steady-state protein levels. The amounts of Myc-tagged Smurf2 plasmid DNA used in transfections are shown in micrograms. (C) Smurf2 increases Smad1 turnover rate; 293 cells transfected with Smad1 and Smurf2 were pulse-labeled with [35S]methionine and then chased for the indicated times. 35S-labeled Smad1 in anti-Smad1 immunoprecipitates was detected by autoradiography of the gel and quantified by phosphorimaging and plotted relative to the amount present at time 0. (D) Ubiquitination of Smad1 and Smad2 in COS-1 cells in the presence of lactacystin. Cell lysates were subjected to anti-HA immunoprecipitation followed by immunoblotting to detect HA-ubiquitin-conjugated Smads. Multi-ubiquitinated species of Smads are indicated (F-Smad-(HA-Ub)n), whereas the lower band may represent an IgG band. Ubiquitination of Smad1 and Smad2 requires the activity of the Smurf2 Hect domain, as the Smurf2 C716G mutant (CG) does not induce ubiquitination of either Smad.