Abstract
The history of advances in the understanding of the toxic effects of lead over the past 20 years is an outstanding example of how knowledge learned from research can impact public health. Measures that have had the greatest impact on reducing exposure to lead are reduction of lead from gasoline, elimination of lead solder from canned food, removal of lead from paint, and abatement of housing containing lead-based paint. Nevertheless, continuing factors that enhance risk to lead exposure, particularly during fetal life, are low socioeconomic status, old housing with lead-containing paint, and less than ideal nutrition, particularly low dietary intake of calcium, iron, and zinc. Prenatal exposure may result from endogenous sources such as lead in the maternal skeletal system or maternal exposures from diet and the environment. Experimental studies have shown that the developing nervous system is particularly sensitive to the toxic effects of lead and that a large number of the effects in the nervous system are due to interference of lead with biochemical functions dependent on calcium ions and impairment of neuronal connections dependent on dendritic pruning. There is need for more study to determine whether these effects are a continuum of prenatal lead exposure or whether prenatal exposure to lead produces unique effects.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfano D. P., Petit T. L. Behavioral effects of postnatal lead exposure: possible relationship to hippocampal dysfunction. Behav Neural Biol. 1981 Jul;32(3):319–333. doi: 10.1016/s0163-1047(81)92372-4. [DOI] [PubMed] [Google Scholar]
- Andrews K. W., Savitz D. A., Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med. 1994 Jul;26(1):13–32. doi: 10.1002/ajim.4700260103. [DOI] [PubMed] [Google Scholar]
- Baghurst P. A., McMichael A. J., Tong S., Wigg N. R., Vimpani G. V., Robertson E. F. Exposure to environmental lead and visual-motor integration at age 7 years: the Port Pirie Cohort Study. Epidemiology. 1995 Mar;6(2):104–109. doi: 10.1097/00001648-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Bellinger D. C. Interpreting the literature on lead and child development: the neglected role of the "experimental system". Neurotoxicol Teratol. 1995 May-Jun;17(3):201–212. doi: 10.1016/0892-0362(94)00081-n. [DOI] [PubMed] [Google Scholar]
- Bellinger D., Leviton A., Waternaux C., Needleman H., Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med. 1987 Apr 23;316(17):1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- Bull R. J., McCauley P. T., Taylor D. H., Croften K. M. The effects of lead on the developing central nervous system of the rat. Neurotoxicology. 1983 Spring;4(1):1–17. [PubMed] [Google Scholar]
- Cookman G. R., Hemmens S. E., Keane G. J., King W. B., Regan C. M. Chronic low level lead exposure precociously induces rat glial development in vitro and in vivo. Neurosci Lett. 1988 Mar 21;86(1):33–37. doi: 10.1016/0304-3940(88)90178-4. [DOI] [PubMed] [Google Scholar]
- Cookman G. R., King W., Regan C. M. Chronic low-level lead exposure impairs embryonic to adult conversion of the neural cell adhesion molecule. J Neurochem. 1987 Aug;49(2):399–403. doi: 10.1111/j.1471-4159.1987.tb02879.x. [DOI] [PubMed] [Google Scholar]
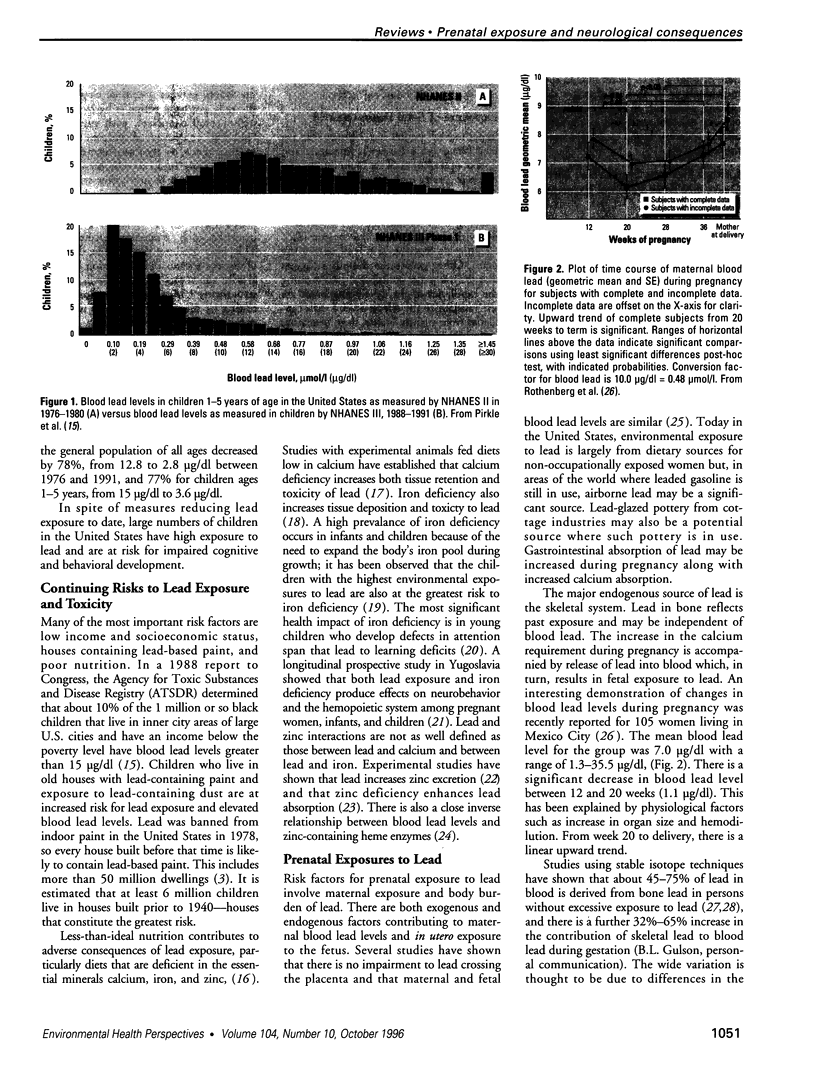
- Cory-Slechta D. A. Bridging human and experimental animal studies of lead neurotoxicity: moving beyond IQ. Neurotoxicol Teratol. 1995 May-Jun;17(3):219–251. doi: 10.1016/0892-0362(94)00085-r. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Otto D. A., Weil D. E., Grant L. D. The comparative developmental neurotoxicity of lead in humans and animals. Neurotoxicol Teratol. 1990 May-Jun;12(3):215–229. doi: 10.1016/0892-0362(90)90093-r. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz B., Dutkiewicz T., Milkowska G. The effect of mixed exposure to lead and zinc on ALA level in urine. Int Arch Occup Environ Health. 1979 Jan 15;42(3-4):341–348. doi: 10.1007/BF00377789. [DOI] [PubMed] [Google Scholar]
- Goyer R. A., Cherian M. G., Jones M. M., Reigart J. R. Role of chelating agents for prevention, intervention, and treatment of exposures to toxic metals. Environ Health Perspect. 1995 Nov;103(11):1048–1052. doi: 10.1289/ehp.951031048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer R. A. Lead toxicity: current concerns. Environ Health Perspect. 1993 Apr;100:177–187. doi: 10.1289/ehp.93100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer R. A. Nutrition and metal toxicity. Am J Clin Nutr. 1995 Mar;61(3 Suppl):646S–650S. doi: 10.1093/ajcn/61.3.646S. [DOI] [PubMed] [Google Scholar]
- Goyer R. A. Transplacental transport of lead. Environ Health Perspect. 1990 Nov;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano J. H., Popovac D., Factor-Litvak P., Shrout P., Kline J., Murphy M. J., Zhao Y. H., Mehmeti A., Ahmedi X., Rajovic B. Determinants of elevated blood lead during pregnancy in a population surrounding a lead smelter in Kosovo, Yugoslavia. Environ Health Perspect. 1990 Nov;89:95–100. doi: 10.1289/ehp.908995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T. R., Miceli R. C., Altmann L., Weinsberg F., Winneke G., Wiegand H. Chronic prenatal and postnatal Pb2+ exposure increases [3H]MK801 binding sites in adult rat forebrain. Eur J Pharmacol. 1993 Oct 1;248(3):273–275. doi: 10.1016/0926-6917(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Gulson B. L., Mahaffey K. R., Mizon K. J., Korsch M. J., Cameron M. A., Vimpani G. Contribution of tissue lead to blood lead in adult female subjects based on stable lead isotope methods. J Lab Clin Med. 1995 Jun;125(6):703–712. [PubMed] [Google Scholar]
- Kelman B. J., Walter B. K. Transplacental movements of inorganic lead from mother to fetus. Proc Soc Exp Biol Med. 1980 Feb;163(2):278–282. doi: 10.3181/00379727-163-40762. [DOI] [PubMed] [Google Scholar]
- Lefauconnier J. M., Bernard G., Mellerio F., Sebille A., Cesarini E. Lead distribution in the nervous system of 8-month-old rats intoxicated since birth by lead. Experientia. 1983 Sep 15;39(9):1030–1031. doi: 10.1007/BF01989787. [DOI] [PubMed] [Google Scholar]
- Mahaffey K. R., Annest J. L. Association of erythrocyte protoporphyrin with blood lead level and iron status in the second National Health and Nutrition Examination Survey, 1976-1980. Environ Res. 1986 Oct;41(1):327–338. doi: 10.1016/s0013-9351(86)80194-3. [DOI] [PubMed] [Google Scholar]
- Markovac J., Goldstein G. W. Lead activates protein kinase C in immature rat brain microvessels. Toxicol Appl Pharmacol. 1988 Oct;96(1):14–23. doi: 10.1016/0041-008x(88)90242-6. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Baghurst P. A., Wigg N. R., Vimpani G. V., Robertson E. F., Roberts R. J. Port Pirie Cohort Study: environmental exposure to lead and children's abilities at the age of four years. N Engl J Med. 1988 Aug 25;319(8):468–475. doi: 10.1056/NEJM198808253190803. [DOI] [PubMed] [Google Scholar]
- Petering H. G. Some observations on the interaction of zinc, copper, and iron metabolism in lead and cadmium toxicity. Environ Health Perspect. 1978 Aug;25:141–145. doi: 10.1289/ehp.7825141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle J. L., Brody D. J., Gunter E. W., Kramer R. A., Paschal D. C., Flegal K. M., Matte T. D. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994 Jul 27;272(4):284–291. [PubMed] [Google Scholar]
- Pollitt E., Hathirat P., Kotchabhakdi N. J., Missell L., Valyasevi A. Iron deficiency and educational achievement in Thailand. Am J Clin Nutr. 1989 Sep;50(3 Suppl):687–697. doi: 10.1093/ajcn/50.3.687. [DOI] [PubMed] [Google Scholar]
- Rice D. C. Behavioral effects of lead in monkeys tested during infancy and adulthood. Neurotoxicol Teratol. 1992 Jul-Aug;14(4):235–245. doi: 10.1016/0892-0362(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Rossouw J., Offermeier J., van Rooyen J. M. Apparent central neurotransmitter receptor changes induced by low-level lead exposure during different developmental phases in the rat. Toxicol Appl Pharmacol. 1987 Oct;91(1):132–139. doi: 10.1016/0041-008x(87)90200-6. [DOI] [PubMed] [Google Scholar]
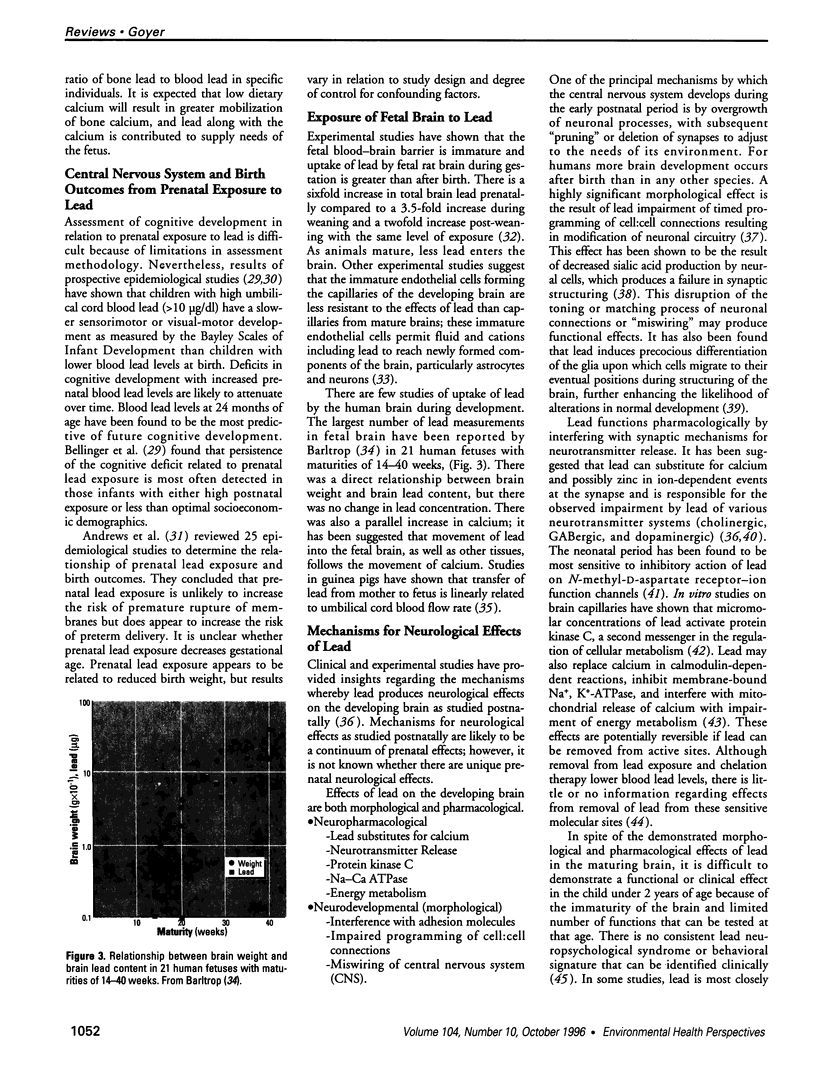
- Rothenberg S. J., Karchmer S., Schnaas L., Perroni E., Zea F., Fernández Alba J. Changes in serial blood lead levels during pregnancy. Environ Health Perspect. 1994 Oct;102(10):876–880. doi: 10.1289/ehp.94102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld E. K. Mechanisms of lead neurotoxicity, or looking beyond the lamppost. FASEB J. 1992 Oct;6(13):3201–3206. doi: 10.1096/fasebj.6.13.1397842. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Cellular interactions between lead and calcium. Br Med Bull. 1986 Oct;42(4):431–434. doi: 10.1093/oxfordjournals.bmb.a072162. [DOI] [PubMed] [Google Scholar]
- Six K. M., Goyer R. A. The influence of iron deficiency on tissue content and toxicity of ingested lead in the rat. J Lab Clin Med. 1972 Jan;79(1):128–136. [PubMed] [Google Scholar]
- Smith D. R., Osterloh J. D., Flegal A. R. Use of endogenous, stable lead isotopes to determine release of lead from the skeleton. Environ Health Perspect. 1996 Jan;104(1):60–66. doi: 10.1289/ehp.9610460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews A. D., Kolber A., Hayward J., Krigman M. R., Morell P. Experimental lead encephalopathy in the suckling rat: concentration of lead in cellular fractions enriched in brain capillaries. Brain Res. 1978 May 19;147(1):131–138. doi: 10.1016/0006-8993(78)90777-1. [DOI] [PubMed] [Google Scholar]
- Wasserman G. A., Graziano J. H., Factor-Litvak P., Popovac D., Morina N., Musabegovic A., Vrenezi N., Capuni-Paracka S., Lekic V., Preteni-Redjepi E. Consequences of lead exposure and iron supplementation on childhood development at age 4 years. Neurotoxicol Teratol. 1994 May-Jun;16(3):233–240. doi: 10.1016/0892-0362(94)90044-2. [DOI] [PubMed] [Google Scholar]