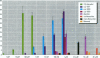Abstract
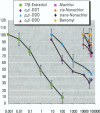
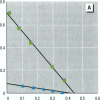
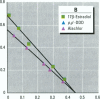
Environmental chemicals that function as estrogens have been suggested to be associated with an increase in disease and dysfunctions in animals and humans. To characterize chemicals that may act as estrogens in humans, we have compared three in vitro assays which measure aspects of human estrogen receptor (hER)-mediated estrogenicity. Chemicals were first tested for estrogen-associated transcriptional activity in the yeast estrogen screen (YES). This was created by expressing hER and two estrogen response elements linked to the lacZ gene in yeast. Second, chemicals that were tested in YES were then assayed for direct interaction with hER in a competition binding assay. Third, chemicals were tested in the estrogen-responsive MCF-7 human breast cancer cell line transiently transfected with a plasmid containing two estrogen response elements linked to the luciferase gene. Together, these assays have identified two metabolites of DDT, o,p'-DDD and p,p'-DDD, that have estrogenic activity. Interestingly, previous studies had reported that the DDD metabolites were nonestrogenic in whole animal models. Alachlor, the most frequently used herbicide in the United States, cis-nonachlor, and trans-nonachlor displayed weak estrogenic activity in the combined assays. The antifungal agent benomyl had no estrogenic activity. We propose that a combination of in vitro assays can be used in conjunction with whole animal models for a more complete characterization of chemicals with estrogenic activity.
Full text
PDF

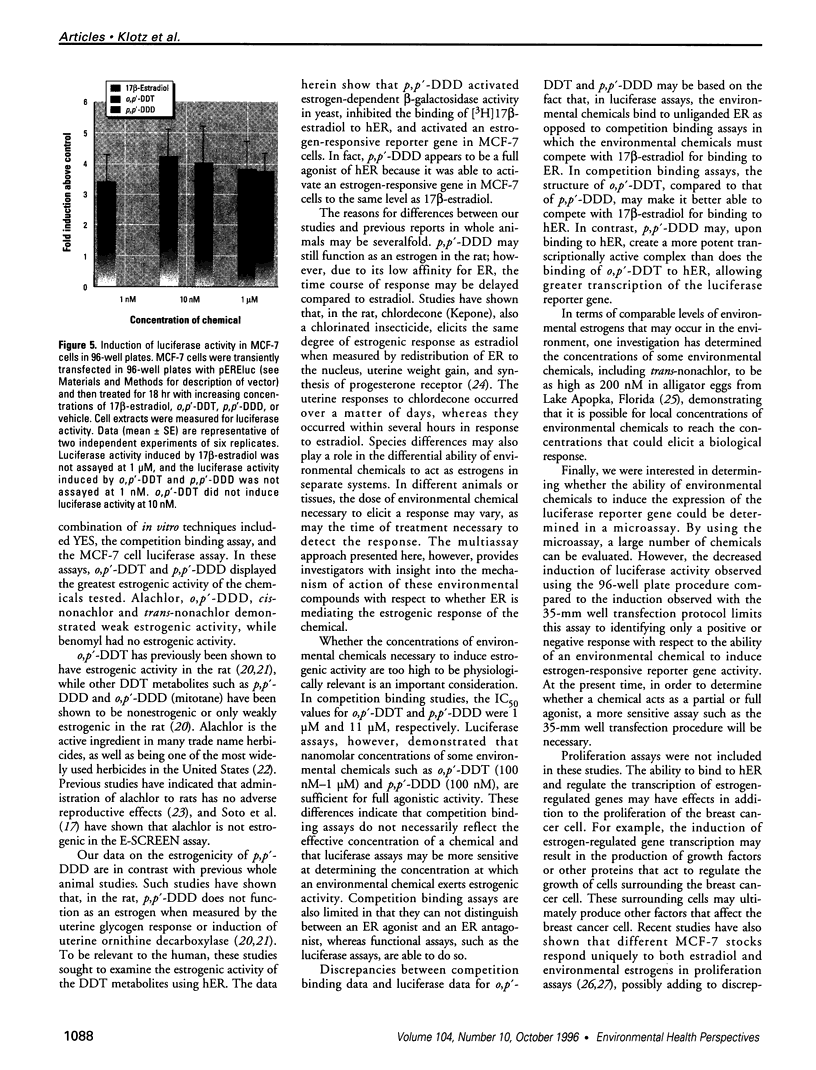

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Robinson M. K., Notides A. C., Guillette L. J., Jr, McLachlan J. A. A yeast estrogen screen for examining the relative exposure of cells to natural and xenoestrogens. Environ Health Perspect. 1996 May;104(5):544–548. doi: 10.1289/ehp.96104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. M., Crews D., McLachlan J. A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994 Sep;102(9):780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitman J., Cecil H. C. Estrogenic activity of DDT analogs and polychlorinated biphenyls. J Agric Food Chem. 1970 Nov-Dec;18(6):1108–1112. doi: 10.1021/jf60172a019. [DOI] [PubMed] [Google Scholar]
- Bitman J., Cecil H. C., Harris S. J., Fries G. F. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968 Oct 18;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck F., Jr, Ricci A., Jr, Wolff M. S., Godbold J., Deckers P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch Environ Health. 1992 Mar-Apr;47(2):143–146. [PubMed] [Google Scholar]
- Fry D. M., Toone C. K. DDT-induced feminization of gull embryos. Science. 1981 Aug 21;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond B., Katzenellenbogen B. S., Krauthammer N., McConnell J. Estrogenic activity of the insecticide chlordecone (Kepone) and interaction with uterine estrogen receptors. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6641–6645. doi: 10.1073/pnas.76.12.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz D. M., Castles C. G., Fuqua S. A., Spriggs L. L., Hill S. M. Differential expression of wild-type and variant ER mRNAs by stocks of MCF-7 breast cancer cells may account for differences in estrogen responsiveness. Biochem Biophys Res Commun. 1995 May 16;210(2):609–615. doi: 10.1006/bbrc.1995.1702. [DOI] [PubMed] [Google Scholar]
- Krieger N., Wolff M. S., Hiatt R. A., Rivera M., Vogelman J., Orentreich N. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst. 1994 Apr 20;86(8):589–599. doi: 10.1093/jnci/86.8.589. [DOI] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A. Effects of dichlorodiphenyltrichloroethane (DDT) analogs and polychlorinated biphenyl (PCB) mixtures on 17beta-(3H)estradiol binding to rat uterine receptor. Biochem Pharmacol. 1974 Jan 15;23(2):447–451. doi: 10.1016/0006-2952(74)90436-5. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos M., Olea N., Brotons J. A., Olea-Serrano M. F., Ruiz de Almodovar J. M., Pedraza V. The E-screen assay: a comparison of different MCF7 cell stocks. Environ Health Perspect. 1995 Sep;103(9):844–850. doi: 10.1289/ehp.95103844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M. S., Toniolo P. G., Lee E. W., Rivera M., Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993 Apr 21;85(8):648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Nagel S. C., Palanza P., Boechler M., Parmigiani S., Welshons W. V. Estrogenic pesticides: binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behaviour in male mice. Toxicol Lett. 1995 May;77(1-3):343–350. doi: 10.1016/0378-4274(95)03316-5. [DOI] [PubMed] [Google Scholar]