Abstract
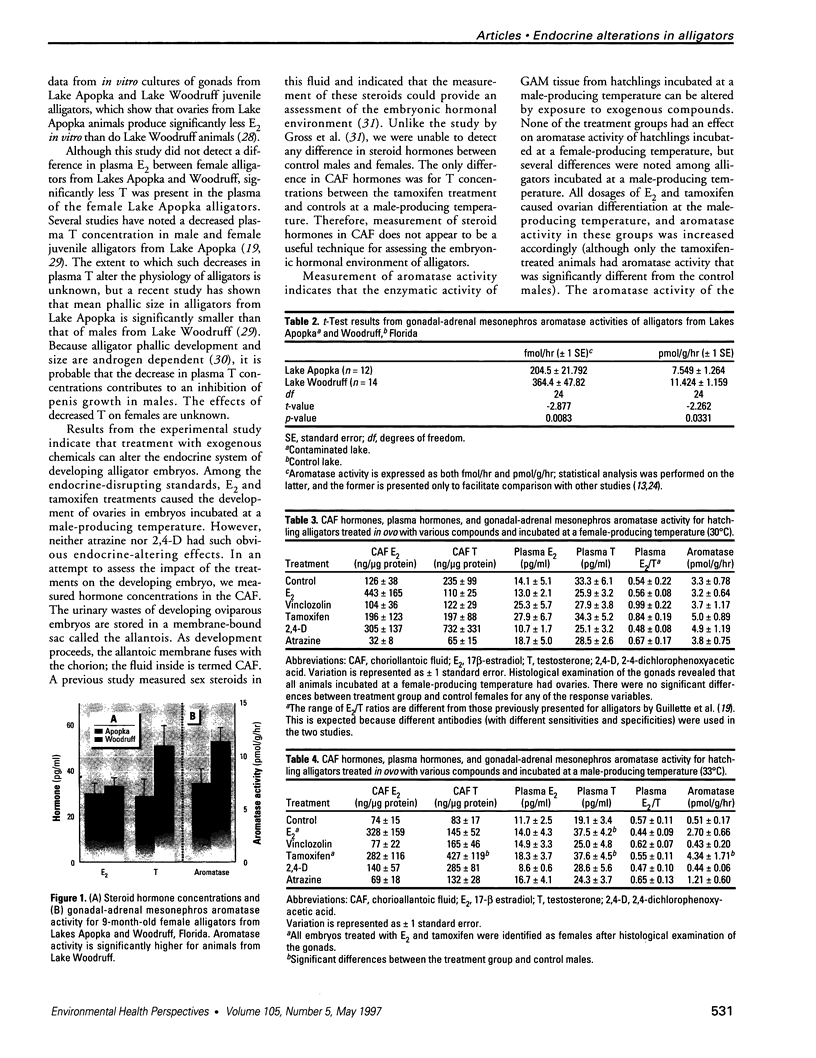
Many environmental contaminants alter the reproduction of animals by altering the development and function of the endocrine system. The ability of environmental contaminants to alter the endocrine system of alligators was studied both in a descriptive study in which juvenile alligators from a historically contaminated lake were compared to animals from a control lake and in an experimental study in which hatchling control alligators were exposed in ovo to several endocrine-disrupting standards and two modern-use herbicides. Endocrine status was assessed by examining plasma hormone concentrations, gonadal-adrenal mesonephros (GAM) aromatase activity, and gonadal histopathology. In the descriptive study, juvenile alligators from the contaminated lake had significantly lower plasma testosterone concentrations (29.2 pg/ml compared to 51.3 pg/ml), whereas plasma 17 beta-estradiol concentrations did not vary when compared to controls. GAM aromatase activity was significantly decreased n the alligators from the contaminated lake (7.6 pmol/g/hr compared to 11.4 pmol/g/hr). In the experimental study, the endocrine-disrupting standards had the expected effects. 17 beta-Estradiol and tamoxifen caused sex reversal from male to female, with a corresponding increase in aromatase activity. Vinclozolin had no apparent effect on male or female alligators. Among the herbicides tested, atrazine induced GAM aromatase activity in male hatchling alligators that was neither characteristic of males nor females, although testicular differentiation was not altered. Exposure to 2,4-dichlorophenoxyacetic acid had no effect on the endocrine parameters that were measured. Together, these studies show that exposure to some environmental chemicals (such as atrazine) can alter steroidogenesis in alligators, but the endocrine alterations previously noted for Lake Apopka, Florida, alligators can not be fully explained by this mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babić-Gojmerac T., Kniewald Z., Kniewald J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J Steroid Biochem. 1989 Jul;33(1):141–146. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- Chardard D., Desvages G., Pieau C., Dournon C. Aromatase activity in larval gonads of Pleurodeles waltl (Urodele Amphibia) during normal sex differentiation and during sex reversal by thermal treatment effect. Gen Comp Endocrinol. 1995 Jul;99(1):100–107. doi: 10.1006/gcen.1995.1089. [DOI] [PubMed] [Google Scholar]
- Connor K., Howell J., Chen I., Liu H., Berhane K., Sciarretta C., Safe S., Zacharewski T. Failure of chloro-S-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam Appl Toxicol. 1996 Mar;30(1):93–101. [PubMed] [Google Scholar]
- Crews D., Bull J. J., Wibbels T. Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol. 1991 Mar;81(3):357–364. doi: 10.1016/0016-6480(91)90162-y. [DOI] [PubMed] [Google Scholar]
- Crews D., Cantú A. R., Bergeron J. M., Rhen T. The relative effectiveness of androstenedione, testosterone, and estrone, precursors to estradiol, in sex reversal in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. Gen Comp Endocrinol. 1995 Oct;100(1):119–127. doi: 10.1006/gcen.1995.1140. [DOI] [PubMed] [Google Scholar]
- Desvages G., Pieau C. Aromatase activity in gonads of turtle embryos as a function of the incubation temperature of eggs. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):851–853. doi: 10.1016/0960-0760(92)90437-n. [DOI] [PubMed] [Google Scholar]
- Elbrecht A., Smith R. G. Aromatase enzyme activity and sex determination in chickens. Science. 1992 Jan 24;255(5043):467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Folmar L. C., Denslow N. D., Rao V., Chow M., Crain D. A., Enblom J., Marcino J., Guillette L. J., Jr Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ Health Perspect. 1996 Oct;104(10):1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T. S., Crain D. A., Bjorndal K. A., Bolten A. B., Carthy R. R. Identification of sex in hatchling loggerhead turtles (Caretta caretta) by analysis of steroid concentrations in chorioallantoic/amniotic fluid. Gen Comp Endocrinol. 1995 Aug;99(2):204–210. doi: 10.1006/gcen.1995.1103. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Crain D. A., Rooney A. A., Pickford D. B. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995 Oct;103 (Suppl 7):157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Gross D. A., Rooney A. A., Percival H. F. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ Health Perspect. 1995 May;103 (Suppl 4):31–36. doi: 10.1289/ehp.95103s431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996 Jan;101(1):32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- Gutzke W. H., Bull J. J. Steroid hormones reverse sex in turtles. Gen Comp Endocrinol. 1986 Dec;64(3):368–372. doi: 10.1016/0016-6480(86)90070-5. [DOI] [PubMed] [Google Scholar]
- Kniewald J., Mildner P., Kniewald Z. Effects of s-triazine herbicides on hormone-receptor complex formation, 5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase activity at the anterior pituitary level. J Steroid Biochem. 1979 Jul;11(1C):833–838. doi: 10.1016/0022-4731(79)90018-9. [DOI] [PubMed] [Google Scholar]
- Kniewald J., Peruzović M., Gojmerac T., Milković K., Kniewald Z. Indirect influence of s-triazines on rat gonadotropic mechanism at early postnatal period. J Steroid Biochem. 1987;27(4-6):1095–1100. doi: 10.1016/0022-4731(87)90195-6. [DOI] [PubMed] [Google Scholar]
- Lance V. A., Bogart M. H. Disruption of ovarian development in alligator embryos treated with an aromatase inhibitor. Gen Comp Endocrinol. 1992 Apr;86(1):59–71. doi: 10.1016/0016-6480(92)90126-5. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Mahendroo M. S., Means G. D., Kilgore M. W., Hinshelwood M. M., Graham-Lorence S., Amarneh B., Ito Y., Fisher C. R., Michael M. D. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994 Jun;15(3):342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Joss J. M. Steroidogenic enzyme activity and ovarian differentiation in the saltwater crocodile, Crocodylus porosus. Gen Comp Endocrinol. 1994 Feb;93(2):232–245. doi: 10.1006/gcen.1994.1027. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Bull J. J., Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J Exp Zool. 1991 Oct;260(1):130–134. doi: 10.1002/jez.1402600117. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Crews D. Putative aromatase inhibitor induces male sex determination in a female unisexual lizard and in a turtle with temperature-dependent sex determination. J Endocrinol. 1994 May;141(2):295–299. doi: 10.1677/joe.0.1410295. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Crews D. Specificity of steroid hormone-induced sex determination in a turtle. J Endocrinol. 1992 Apr;133(1):121–129. doi: 10.1677/joe.0.1330121. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Gideon P., Bull J. J., Crews D. Estrogen- and temperature-induced medullary cord regression during gonadal differentiation in a turtle. Differentiation. 1993 Jul;53(3):149–154. doi: 10.1111/j.1432-0436.1993.tb00703.x. [DOI] [PubMed] [Google Scholar]