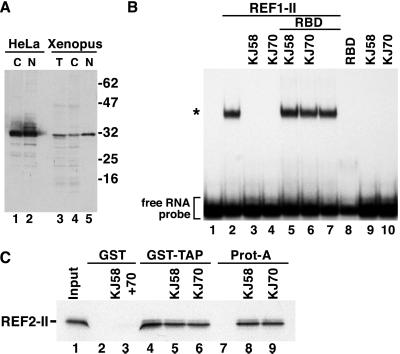
Figure 1.
Anti-REF antibodies prevent REF binding to RNA. (A) Protein samples from HeLa cytoplasmic (C) or nuclear extracts (N), total Xenopus oocytes (T), or cytoplasmic (C) and nuclear (N) fractions were analyzed by Western blot using anti-REF antibodies. (B) An electrophoretic mobility retardation assay was performed with a labeled RNA probe and recombinant GST-REF1-II protein (20 ng/μl). Lane 1, free RNA; lane 2, REF/RNA complexes; lanes 3 and 4, when affinity-purified REF antibodies (KJ58 and KJ70) were included in the reaction, formation of REF/RNA complexes was prevented. The inhibitory effect was abolished if purified GST-RBD was added together with the antibodies to the binding reaction (lanes 5 and 6). Controls show that the RBD fusion and the antibodies on their own have no effect on RNA mobility (lanes 7–10). The concentration of the antibodies in the binding reactions was 0.5 mg/ml and that of GST-RBD 1 mg/ml. The position of the free RNA probe (lane 1) and of the REF/RNA complexes (*) is shown on the left. (C) 35S-methionine-labeled REF2-II was synthesized in vitro in rabbit reticulocyte lysates. Ten-microliter aliquots were incubated with affinity-purified antibodies. After a 20-min incubation period, samples were divided and assayed by binding to glutathione agarose beads precoated with GST-TAP or to protein-A Sepharose beads. Bound fractions were analyzed by SDS/PAGE and fluorography.