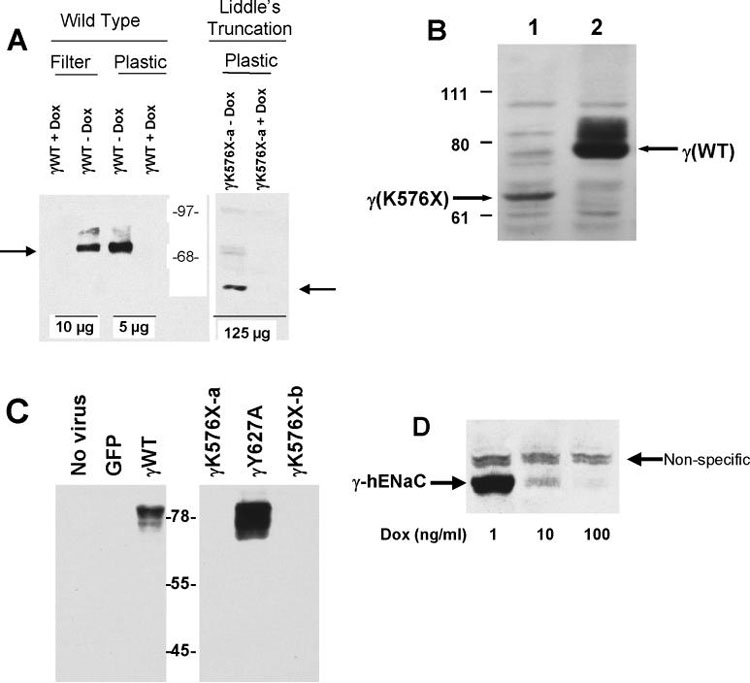
Figure 2:
A. Immunoblot of γ-hENaC wild type (WT) and C-terminal (Liddle's) truncations. All constructs infected at 10 MOI in M1 cells with 10 MOI tTA with or without 1,000 ng/ml doxycycline (DOX) as indicated. Amount of protein loaded per lane indicated at bottom. Arrows indicate location of full-length γhENaC (left) and the Liddle's truncation (γK576X, right). Molecular standards in center. B. Immunoblot of wild type (lane 2) or Liddle's truncated (lane 1) γhENaC constructs infected into M1 cells grown on plastic as in 2A without doxycycline. This immunoblot used a sheep antibody directed at the N-terminus of γhENaC (21). 100 μg protein loaded in each lane. C. Immunoblot of γhENaC constructs infected into M1 cells (10 MOI) grown on filters without doxycycline. Left panel shows no evidence of immunoreactive material in negative controls while showing abundant material in cells infected with 10 MOI wild type γhENaC construct. Right panel shows no detectable γhENaC for the Liddle's truncation (γK576X). In contrast, the γY627A mutant shows abundant protein. 70 μg protein loaded in each lane. All cells grown on filters. D. Immunoreactive γhENaC protein decreases with increasing concentration of doxycycline (DOX) in the medium. 100 μg total protein loaded per lane. Non-specific band seen with heavy protein loading.