FIGURE 4.
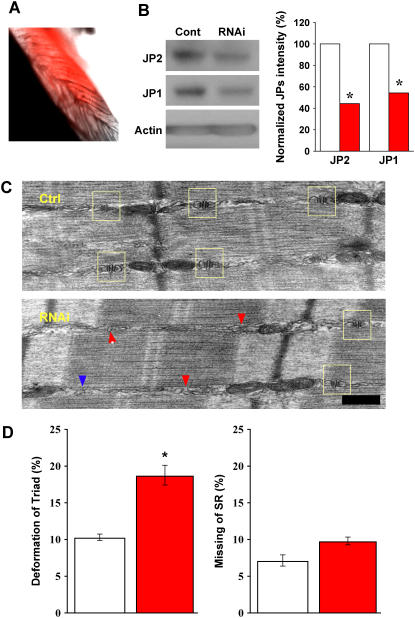
Suppression of JP1 and JP2 leads to defective triad junction in skeletal muscle. (A) A typical sample of FDB muscle infected with adenovirus packaged with shRNA against JP1 and JP2, taken 4 days postinjection of virus. Red color represents RFP fluorescence, indicating infected fibers. (B) Western blot of JP1 and JP2 expression in skeletal muscle fibers, 4 days postinfection with adenoviruses packaged with nonspecific shRNA (control) and specific shRNA against JP1 and JP2 (RNAi). Similar to results in C2C12 cells, normalized protein levels with actin show that shRNAs significantly knock down both JP1 and JP2. (C) Defective triad junction in skeletal muscle, examined by EM. Top panel shows a typical muscle fiber from the Ad-cont-infected mice, and the bottom panel shows a typical muscle fiber from the Ad-shRNA-infected mice. Scale bar indicates 0.2 μm. The sides of A-I junctional regions of myofibrils where triads are expected to be present were observed. In the case where normal paired triad junctions (yellow box) flanking each Z-disk could not be observed by eye, such regions were assigned “deformed triad”. TT and SR without coupling were seen in the “deformed triad”, and such membrane structures were not seen very frequently at the regions analyzed (<20% in all counted). Muscle infected with Ad-cont resembles wild-type, with normal SR/TT/SR architecture (yellow box), whereas in Ad-shRNA-infected muscle triad junctions are frequently deformed (red arrow) or missing (blue arrow). (D) Bar graph shows the summarized data obtained from the EM analyses of more than 1000 A-I junctional regions from 11 muscle specimens obtained from 6 different animals. p = 0.00381 (left panel) and 0.0453 (right panel). To insure accuracy in morphological assessment, we increased stringency for statistical significance. p-value <0.01 was considered statistical significance. Data presented as mean ± SE.