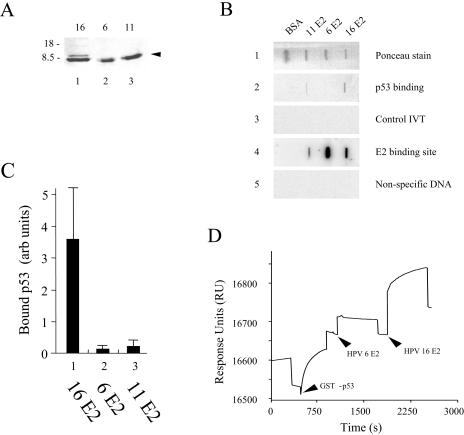
FIG. 4.
LR-HPV6 and LR-HPV11 E2 bind poorly to p53. (A) Samples of the purified HR-HPV16 E2Ct, LR-HPV6 E2Ct, and LR-HPV11 E2Ct proteins were analyzed by SDS-PAGE (indicated by the arrowhead). The sizes of the markers used are indicated to the left of the gel. (B) Equal amounts (250 pM) of the purified HR-HPV16 E2Ct, LR-HPV6 E2Ct, and LR-HPV11 E2Ct proteins were immobilized on a PVDF membrane by slot blotting. The membrane was then incubated with 35S-labeled p53, and after extensive washing, the bound protein was visualized using a PhosphorImager (panel 2). After washing to remove the bound p53, the membrane was incubated with an unrelated 35S-labeled protein (panel 3). A duplicate membrane was incubated with a 32P-labeled 20-bp oligonucleotide carrying an E2 binding site (panel 4) or a 32P-labeled unrelated oligonucleotide (panel 5), and bound DNA was visualized as described above. (C) The graph shows the results of three filter-binding experiments performed in duplicate. The data shown are averages and standard deviations. (D) SPR analysis of LR-HPV6 E2Ct and HR-HPV16 E2Ct binding to immobilized GST-p53. A GST-p53 fusion protein was immobilized through amine coupling on a CM5 sensor chip. LR-HPV6 E2Ct was then passed over the immobilized GST-p53. After reequilibration, HR-HPV16 E2Ct was passed over the immobilized GST-p53. As can be seen from the data, HR-HPV16 E2Ct brings about a significant increase in the SPR signal, whereas LR-HPV6 E2Ct has little or no effect.