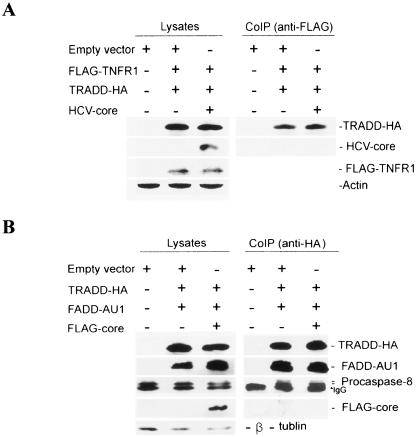
FIG. 2.
HCV core protein does not interfere with TNFR1-TRADD or TRADD-FADD-procaspase-8 interaction. (A) In vivo coimmunoprecipitation of TNFR1 and TRADD in the presence of HCV core protein. HepG2 cells were infected with vvT7 and cotransfected with empty vector or with FLAG-TNFR1, TRADD-HA, and core expression plasmids under the control of a T7 promoter. After 24 h of transfection, cell lysates were immunoprecipitated with a mouse monoclonal antibody to FLAG for TNFR1 and immunoblotted with an HA-specific antibody for TRADD, FLAG for TNFR1, and a rabbit antiserum for the core protein. The expression of HCV core protein and FLAG-TNFR1 was verified by immunoblotting cell lysates. The level of cellular actin was used as an internal control for comparison. (B) In vivo coimmunoprecipitation of TRADD-FADD-procaspase-8 in the presence of HCV core protein. HepG2 cells were infected with vvT7 and cotransfected with TRADD-HA, FADD-AU1, and FLAG-core expression plasmids. Anti-HA antibody and protein G-Sepharose beads were added to cell lysates for immunoprecipitation of the TRADD-FADD-procaspase-8 complex. The immunoprecipitate was subjected to Western blot analysis for detection of TRADD-HA, FADD-AU1, procaspase-8, and FLAG-core with specific antibodies. The presence of TRADD-HA, FADD-AU1, FLAG-core proteins, and endogenous procaspase-8 in cell lysates was verified by immunoblotting. The level of cellular tubulin was used as an internal control for comparison. The molecular weight of the protein bands were ascertained from the migration of standard protein molecular weight markers (Bio-Rad).