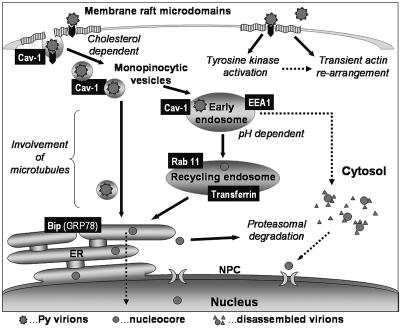
FIG. 9.
Scheme of trafficking pathways used by PyV for the movement towards the cell nucleus. Virions are internalized through lipid raft domains into smooth, often caveolin-positive, monopinocytic vesicles. These vesicles then fuse with early endosomes, and endosomal acidic pH is (in contrast to SV40) necessary for efficient PyV trafficking. Virus uptake is accompanied with protein tyrosine kinase signaling (our unpublished observation) and with a transient reorganization of the actin network (33). Further movement of the virus is directed into the recycling endosomes, as we proved by FRET between PyV VP1 and transferrin or between PyV VP1 and Rab11 GTPase. Significant colocalization of VP1 with the BiP marker was observed previously (20) and suggests that PyV can be transported to the ER. However, in contrast to cholera toxin, or to SV40, the PyV route into the ER is independent of COPI vesicles (20). We also detected direct fusions of virus-carrying monopinocytic vesicles with the ER on ultrathin cell sections (33); therefore, both routes to the ER (the one from recycling endosomes and the direct one from monopinocytic vesicles) are considered alternative routes. The possibility that “successful” virions escape from early endosomes into the cytosol (as a result of conformation changes induced by acidic pH) still cannot be ruled out. The trafficking pathway leading to the ER via recycling endosomes would then represent a cell defense mechanism. We have shown previously (20) that only a few viral genomes reach the nucleus, while a substantial amount is directed to the ER-associated degradation pathway. It is conceivable that PyV misuses this pathway, like some bacterial toxins (10), counting upon an escape of several viral genomes into the nucleus prior to their degradation in the cytoplasm. Black arrows, experimentally proved routes; dotted arrows, possible alternative routes. NPC, nuclear pore complex.