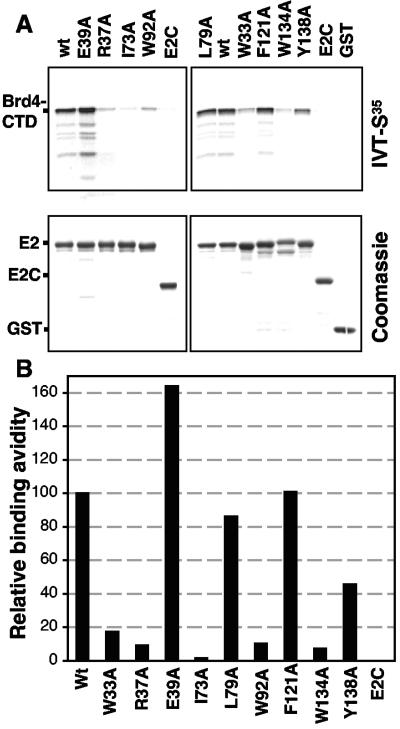
FIG. 1.
Binding of HPV16 E2 transactivation domain mutants to the Brd4 C-terminal domain. (A) GST-tagged E2 (wild type or mutant) was expressed and purified from E. coli. Equal amounts of the proteins were incubated with in vitro-translated (IVT) Brd4-CTD and glutathione-Sepharose beads. After extensive washings, the proteins were separated by SDS-polyacrylamide gel electrophoresis. wt E2 served as the positive control, and the C-terminal 171 amino acids of E2 (E2C) and GST alone (GST) served as negative controls. Upper panel, autoradiogram of 35S-labeled Brd4-CTD bound to the indicated GST-E2 fusion protein; lower panel, Coomassie-stained gel of the input GST fusion proteins used in the binding experiment. (B) The bound 35S-labeled Brd4-CTD was quantified with a PhosphorImager, with wt E2 binding set to 100%.