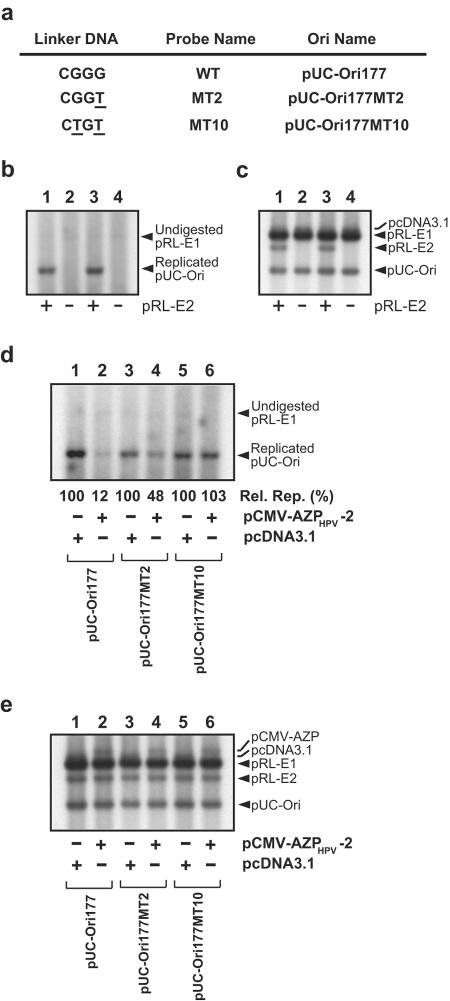
FIG. 6.
Transient replication of HPV-18 mutant ori plasmids with AZPHPV-2. (a) Mutant probes and mutant ori plasmids. The mutations were introduced into the 4-bp spacer DNA (i.e., 5′-CGGG-3′) located between E2BS-3 and E2BS-4 to minimize any negative effect on HPV-18 DNA replication due to the mutations. (b) Importance of E2 for mutant ori replication. A transient replication assay was performed with pRL-E1 for E1 expression and pUC-Ori177M2 (lanes 1 and 2) or pUC-Ori177M10 (lanes 3 and 4) as a mutant ori plasmid in the presence of the E2 expression plasmid pRL-E2 (lanes 1 and 3) or in the absence of pRL-E2 (lanes 2 and 4). Hirt-extracted samples were digested with DpnI to eliminate unreplicated input plasmids. (c) Examination of amounts of plasmids introduced into 293H cells for panel b. This panel shows Southern blot hybridization with Hirt-extracted DNA samples which were not treated with DpnI, indicating relative amounts of plasmids introduced into 293H cells. (d) Replication of HPV-18 mutant ori plasmids in the presence of pCMV-AZPHPV-2. Transient replication was assayed with pRL-E1, pRL-E2, and a pUC-Ori177 derivative along with pCMV-AZPHPV-2 or pcDNA 3.1, as indicated below each lane. Hirt-extracted samples were digested with DpnI. Efficiencies of inhibition of DNA replication by AZPs were judged by comparison with relative amounts of each pUC-Ori177 derivative. The amount of each replicated pUC-Ori177 derivative in the presence of AZPHPV-2 was compared with that in the absence of AZP (100%). The average data [Rel. Rep. (%)] from four independent assays are indicated below each lane. (e) Examination of amounts of plasmids introduced into 293H cells for panel d. This panel shows Southern blot hybridization with Hirt-extracted DNA samples which were not treated with DpnI, indicating relative amounts of plasmids introduced into 293H cells.