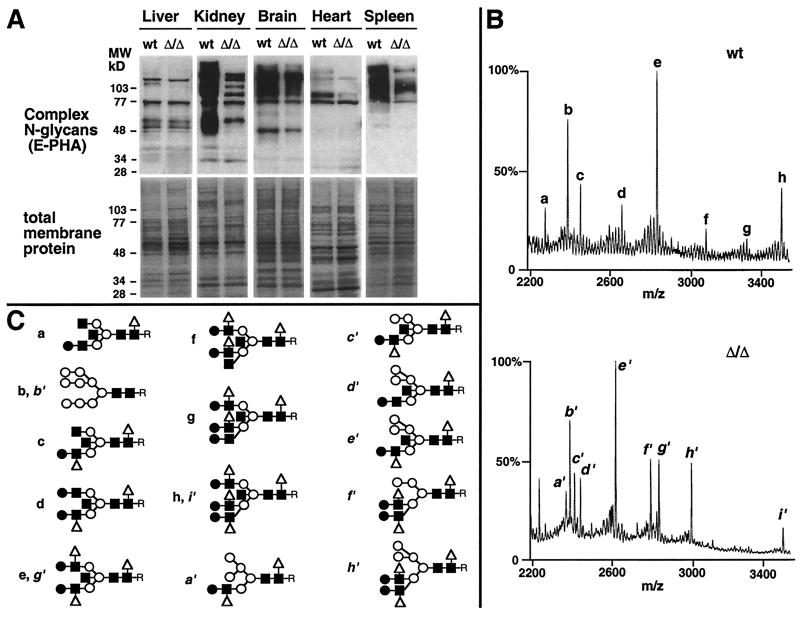
Figure 2.
Reduction in complex N-glycans and increased hybrid N-glycan structures in the absence of α-mannosidase II. (A) Complex N-glycans are deficient on glycoproteins from some tissues in mice homozygous for a deletion in the α-mannosidase II gene (Δ/Δ). Membrane protein was isolated from various tissues, and complex N-glycans were visualized by binding to E-phytohemagglutinin (Upper) as previously described (14). Equivalent amounts of membrane protein were used in the analyses (Lower). (B) Mass spectrometry of N-glycans from various tissues (kidney shown) was accomplished after isolation from glycoproteins by PNGase F. Subsequent treatment with various glycosidases (not shown) provided additional information on specific saccharide linkages (16). (C) N-Glycan structures (desialylated) defined by mass spectrometry from wild-type tissues were mostly complex types with fully modified mannose termini bearing N-acetylglucosamine linkages (a, c–h), whereas structures in the absence of α-mannosidase II contained hybrid N-glycans noted by terminal mannose residues (a′, c′, d′, e′, f′, h′). The anomeric glycosidic linkages among the core regions are indicated (Fig. 1). Antennary extensions are with β1–2-linked glucosamine, β1–4-linked galactose, and α1–6-linked fucose and are as described for the relevant Lewis antigens (38). R indicates the position of the asparagine residue before release of N-glycans from glycoproteins by PNGase F. For monosaccharide symbols, see Fig. 1 legend.