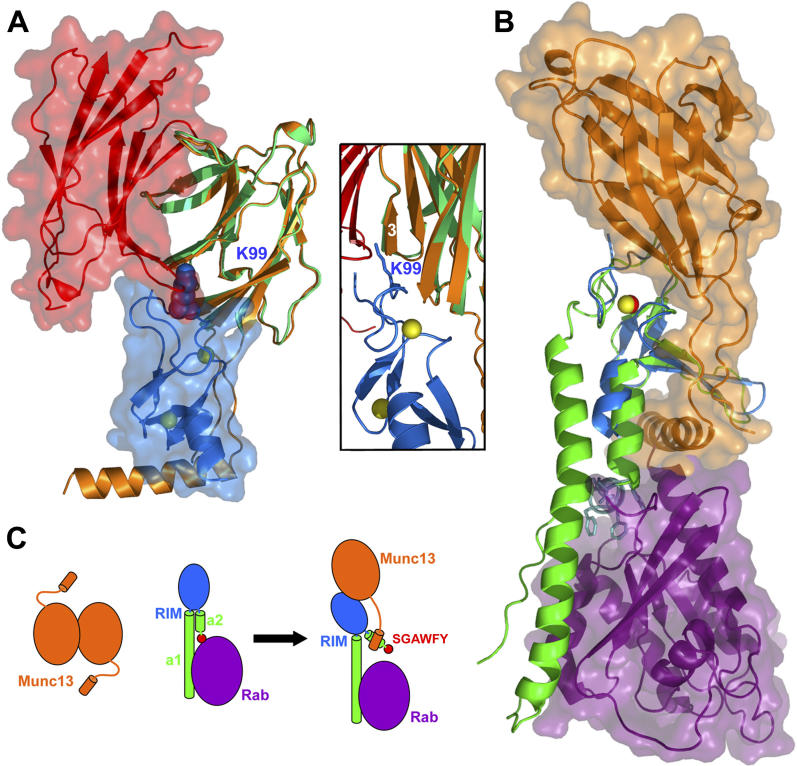
Figure 7. Interplay between the Interactions of Munc13–1, α-RIM, and Rab3s.
(A) Structures of the Munc13–1 C 2A-domain homodimer and the Munc13–1 3–150(K32E)/RIM2α 82–142 heterodimer superimposed using monomer C of the homodimer (green) and monomer A of the heterodimer (orange). Ribbon diagrams and transparent surface representations are shown for monomer A of the homodimer (red) and RIM2α 82–142 (blue) to illustrate the steric clash that would occur if both complexes co-existed. The K99 side chain of the ZF domain is shown as a space-filling model. The inset shows a close-up of the region where this side chain (shown here as stick model) would insert into the homodimer interface (the surface representations are not shown).
(B) Structures of the Munc13–1 3–150(K32E)/RIM2α 82–142 heterodimer and the rabphilin/Rab3A complex [ 27] superimposed using the ZF domains of RIM2α (blue) and rabphilin (green). Ribbon diagrams and transparent surface representations are shown for Munc13–1 3–150(K32E) (orange) and Rab3A (purple). Zinc atoms are shown as yellow (RIM2α) or red (rabphilin) spheres. The SGAWFF motif of rabphilin is shown as cyan stick model.
(C) Model of a cascade of protein–protein interactions that may regulate synaptic vesicle priming and presynaptic plasticity. Munc13–1 is shown in orange and Rab3 in purple. α-RIM is shown in blue (ZF domain), green (helices a1 and a2), and red (SGAWFY motif). The model represents how formation of the tripartite Munc13–1/α-RIM/Rab3 complex involves dissociation of the Munc13–1 homodimer and release of the interaction between the SGAWFY motif and Rab3A.