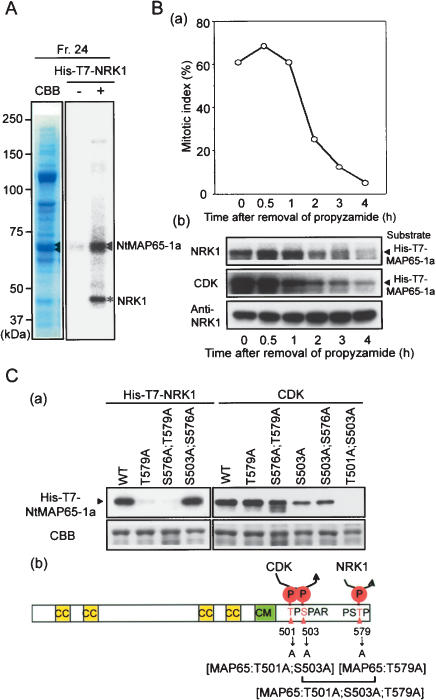
Figure 1.
Phosphorylation of NtMAP65-1a by NRK1/NTF6 MAPK in vitro. (A) Phosphorylation in vitro of MAPs by NRK1. MAPs were prepared from tobacco BY-2 cells, and each MAPs fraction was used as substrate in a kinase assay with or without His-T7-NRK1 in the presence of [γ-32P]ATP. (Right) NRK1-phosphorylated proteins were detected by autoradiography in fraction 24. (Left) The same fraction was stained with Coomassie Brilliant Blue (CBB). The arrowheads and asterisk show the identified phosphorylated proteins and His-T7-NRK1, respectively. (B) Phosphorylation of NtMAP65-1a by endogenous NRK1/NTF6 and CDKs during the M-to-G1 transition. (Panel a) Mitotic indices at the indicated times after removal of propyzamide. (Panel b) Phosphorylation by NRK1/NTF6 and CDKs of His-T7-NtMAP65-1a during the M phase in BY-2 cells. Proteins were extracted from cells that had been harvested at the indicated time after removal of propyzamide. Kinase activities of NRK1/NTF6 (top) and CDKs (middle) were determined by an immunocomplex kinase assay and a p13SUC1 beads complex kinase assay, respectively, with His-T7-NtMAP65 as substrate. (Bottom) Proteins extracted from BY-2 cells were also analyzed by immunoblotting with NRK1-specific antibodies (anti-NRK1; (Soyano et al. 2003). (C) Identification of sites of phosphorylation in NtMAP65-1a by NRK1 and CDKs. (Panel a) Kinase assays in vitro using recombinant NRK1 (left) and CDKs prepared from BY-2 cells with p13SUC1 beads (right). Recombinant NtMAP65-1a protein and mutant proteins in which serine or threonine residues at positions 501, 503, 576, and/or 579 have been replaced by alanine in various combinations were used as substrates. (Panel b) A schematic representation of NtMAP65-1a. The coiled-coil motif (CC) and the motif (CM) that is conserved in the MAP65 family are shown as yellow and green boxes, respectively. Sites of phosphorylation (P) by NRK1 and CDKs and the amino acid substitutions in the various mutant forms of NtMAP65-1a are indicated.