Abstract
Wolbachia spp. are obligate maternally inherited endosymbiotic bacteria that infect diverse arthropods and filarial nematodes. Previous microscopic and molecular studies have identified Wolbachia in several bed bug species (Cimicidae), but little is known about how widespread Wolbachia infections are among the Cimicidae. Because cimicids of non-medical importance are not commonly collected, we hypothesized that preserved museum specimens could be assayed for Wolbachia infections. For the screening of museum specimens, we designed a set of primers that specifically amplify small diagnostic fragments (130 to 240 bp) of the Wolbachia 16S rRNA gene. Using these and other previously published primers, we screened 39 cimicid species (spanning 16 genera and all 6 recognized subfamilies) and 2 species of the sister family Polyctenidae for Wolbachia infections using museum and wild-caught material. Amplified fragments were sequenced to confirm that our primers were amplifying Wolbachia DNA. We identified 10 infections, 8 of which were previously undescribed. Infections in the F supergroup were common in the subfamily Cimicinae, while infections in the A supergroup were identified in the subfamilies Afrocimicinae and Haematosiphoninae. Even though specimens were degraded, we detected infections in over 23% of cimicid species. Our results indicate that Wolbachia infections may be common among cimicids and that archived museum material is a useful untapped resource for invertebrate endosymbiont surveys. The new screening primers listed in this report will be useful for other researchers conducting Wolbachia surveys with specimens with less-than-optimum DNA quality.
Wolbachia spp. are endosymbiotic bacteria that have been described with a diverse range of arthropods and filarial nematodes (8, 11, 18, 19, 21, 25, 27, 28). Eight major Wolbachia “supergroups” (A to H) exist based on phylogenetic clustering of FtsZ gene sequences (11). A, B, and E infect diverse arthropods; C and D infect nematodes; G infects spiders; H infects termites; and F infects both arthropods and nematodes (4, 5, 8, 11, 18, 19, 21, 25, 27, 28). Wolbachia infections are commonly associated with diverse host reproductive alterations, including cytoplasmic incompatibility, feminization, male killing, parthenogenesis, increased or decreased fitness, and obligate symbiosis (21). Because of the phenotypes induced by these infections, it has been suggested that the manipulation of endosymbiotic bacteria can be used as a novel method for the biocontrol of pest arthropods of medical, veterinary, and agricultural importance (3, 16, 17, 20, 22, 29, 30).
The Cimicidae (bed bugs) are obligatory hematophagous ectoparasites of birds, bats, and humans (24). Wolbachia-like bacterial inclusions were observed several decades ago in the gonads, spermalege (i.e., organ of Berlese), gut, Malpighian tubules, and hemolymph of the cimicids Cimex lectularius and Oeciacus hirundinis (1, 24). Additionally, similar organisms have been described from the bacteriomes (i.e, mycetomes) of C. lectularius (5, 26). More recently, modern molecular methods were used to conclusively identify Wolbachia symbionts in C. lectularius and Oeciacus vicarius (8, 18), which were determined to be closely related to one another in the F supergroup (11, 18). However, except for these two species, nothing is known about the distribution of Wolbachia infections among the family Cimicidae.
We undertook a PCR-based survey to screen for Wolbachia infections in the Cimicidae. Studies of this nature are complicated by the fact that, apart from species of medical importance, cimicids that feed on nonhuman hosts are not frequently collected. Non-medically important cimicids are obtained primarily incidentally during vertebrate ectoparasite surveys and are often preserved in ethanol and archived in museum collections. Museum specimens have previously been used for molecular surveys of bacteria, such as Borrelia, Helicobacter, and Mycobacterium spp. (2, 7, 9, 12, 13, 15). We therefore hypothesized that ethanol-preserved museum material could be used in a similar manner for Wolbachia surveys. Despite degraded DNA in many specimens, it was possible to amplify and sequence diagnostic fragments of the Wolbachia 16S rRNA gene in both wild-caught and preserved cimicid specimens. In a screen of 39 species of Cimicidae and 2 species in the sister family Polyctenidae (24), 10 Wolbachia infections were identified, 8 of which were previously undescribed. Wolbachia infections were detected in ethanol-preserved museum specimens up to 48 years old. Our results suggest that Wolbachia infections may be common in the family Cimicidae and that museum collections can act as a valuable untapped resource for molecular surveys for invertebrate endosymbionts.
MATERIALS AND METHODS
Insect samples.
Assayed specimens and collection information are listed in Table 1. Wild specimens were collected from vertebrate hosts or from dwellings, placed into either 100% ethanol (Afrocimex constrictus) or dried with silica desiccant (Cimex lectularius), and transported to the Johns Hopkins Bloomberg School of Public Health for further processing. Museum specimens came either from private donors or from the Cimicidae collection compiled by Robert Leslie Usinger, a collection of over 10,000 cimicid specimens stored in ethanol and housed at the Essig Museum of Entomology, University of California, Berkeley. The Usinger collection specimens date from 1966 or earlier. All museum specimens had been stored in 95 to 100% ethanol since their collection date (Table 1) and were processed in a manner similar to that used for ethanol-preserved wild material.
TABLE 1.
Collection information and sample sizes for specimens assayed in this study
| Species | No. of specimens | Source | Collection date | Collection locality | Referenceb |
|---|---|---|---|---|---|
| Afrocimex constrictus | 5e | Wildf | March 2005 | Mt. Elgon, Kenya | |
| Aphrania vishnou | 2e | Museumg | 1952a | Phnom Penh, Cambodiaa | 24 |
| Bucicimex chilensis | 1e | Museumg | January 1962 | Lab colony of unknown origin | |
| Cacodmus vicinus | 1e | Museumg | April 1959 | Giza, Egypt | |
| Cimex adjunctus | 2e | Museumf | July 2001 | Hillsboro County, N.H. | |
| Cimex antennatus | 6e | Museumg | July 1963 | Siskiyou County, Calif. | |
| Cimex brevis | 6e | Museumg | Before 1966a | Staples, Minn. | 24 |
| Cimex columbarius | 2e | Museumg | July 1958a | Island of Korpo, Finlanda | 23, 24 |
| Cimex hemipterus | 2e | Museumg | Before 1966a | Taiwan | 24 |
| Cimex incrassatus | 2e | Museumf | July 1997 | Orange County, Calif. | |
| Cimex insuetus | 8e | Museumg | Before 1966a | Saraburi, Thailand | 24 |
| Cimex latipennis | 8e | Museumg | Before 1966a | Klamath Lake, Oreg. | 24 |
| Cimex lectularius | 12d | Wildf | Jan-Feb 2005 | Lupata, Macha, Zambia | |
| Cimex pilosellus | 7e | Museumf | July 1994 | Pend-d'Oreille Valley, British Columbia, Canada | |
| Cimex pipistrelli | 7e | Museumg | Before 1966a | Lab colony derived from England | 24 |
| Cimex stadleri | 6e | Museumg | June 1956 | Bmo, Czechoslovakiaa | 24 |
| Haematosiphon inodorus | 2e | Museumf | July 1976 | Presidio County, Tex. | |
| Hesperocimex cochimiensis | 3e | Museumg | July 1957 | Baja California, Mexico | |
| Hesperocimex coloradensis | 8e | Museumf | July 1971 | Los Alamos County, N.Mex. | |
| Hesperocimex sonorensis | 8e | Museumg | January 1958 | Lab colony of unknown origin | |
| Hesperoctenes eumops | 1e | Museumg | June 1945 | Fresno County, Calif. | |
| Hesperoctenes fumarius | 2e | Museumf | August 2003 | St. John, U.S. Virgin Islands | |
| Latrocimex sp. | 4e | Museumg | October 1957 | Trinidad | |
| Leptocimex boueti | 1e | Museumg | August 1962 | Ivory Coast | |
| Leptocimex duplicatus | 1e | Museumg | Before 1966a | Lab colony derived from Egypt | |
| Loxapsis malayensis | 6e | Museumg | December 1962 | Tasik Bera, Pahang, Malaysia | |
| Oeciacus hirudinis | 19e | Museumg | July 1960 | Faraya, Lebanon | |
| Oeciacus vicarius | 1e | Museumf | July 2004 | Oconee County, S.C. | |
| Ornithocoris furnarii | 9e | Museumg | January 1958 | Lab colony of unknown origin | |
| Ornithocoris pallidus | 1e | Museumg | 1969 | Hancock County, Miss | |
| Ornithocoris toledoi | 5e | Museumg | 1957 | Ponte Nova, Brazil | |
| Paracimex borneensis | 10e | Museumg | November 1966 | Fraser's Hill, Malaysia | |
| Paracimex caledoniae | 5e | Museumg | March 1945 | New Caledonia | |
| Paracimex capitatus | 11e | Museumg | July 1966 | Edie Creek, Papua New Guinea | |
| Paracimex gerdheinrichi | 3e | Museumg | May 1966 | Rantepao, Indonesia | |
| Paracimex inflatus | 7e | Museumg | July 1966 | Kavieng, Papua New Guinea | |
| Paracimex reductus | 6e | Museumg | January 1962 | Kebar Valley, New Guinea | |
| Paracimex setosus | 5e | Museumg | February 1962a | Sarangan, Java, Indonesia | 24c |
| Primicimex cavernis | 1e | Museumg | March 1967 | Lake Patzaquaro, Mexico | |
| Psitticimex uritui | 1e | Museumf | February 1990 | La Pampa, Argentina | |
| Stricticimex transversus | 4e | Museumg | October 1957 | Kanye, Botswana |
Data lacking on collection label; information inferred from other sources, if possible.
Data are based on additional information in reference(s) indicated.
Collection date illegible on label; correct date located in reference 24.
Specimens dessicated.
Specimens in ethanol.
Private donor.
Usinger Collection, Essig Museum of Entomology, University of California, Berkeley, Calif.
DNA extraction.
In most cases, we were constrained by the specimen donor in terms of the number of samples that could be processed for DNA extraction. Sample sizes are listed in Table 1. To preserve the external morphology of processed insects and, thus, their value as museum specimens, we used a minimally destructive method for DNA extraction. We tested two variants of the extraction protocol, one based on DNeasy spin columns (QIAGEN, Valencia, CA) and the other based on high salt-ethanol extraction (16). For both protocols, insect abdomens were cut with a sterile razor or punctured several times with a fine needle (for small specimens). For the QIAGEN protocol, specimens were digested overnight (∼18 h) in 180 μl 1× phosphate-buffered saline, 20 μl proteinase K, and 200 μl AL buffer solution. The digestate was vortexed with 200 ml of 100% cold ethanol, applied onto DNeasy columns, and DNA bound, washed, and eluted according to the manufacturer's suggested protocol. For the salt extraction protocol, specimens were digested in extraction buffer for ∼18 h, and the digestate was processed as described previously (16). After the digestions, the exoskeletons were removed, placed in 100% ethanol, and archived at −20°C. Some specimens were mounted permanently on glass slides using Euparal permanent mounting medium (Bioquip Products, Rancho Dominguez, CA). We found that the salt extraction protocol tended to result in higher yields of extracted DNA but observed no differences in PCR success between the two protocols.
Wolbachia-specific PCR assays.
All PCRs were conducted using Cimex lectularius colony specimens known to be infected as a positive control and a reaction containing all PCR ingredients except template DNA as a negative control. Specimens were assayed individually. Each 25-μl reaction consisted of 1 μl template DNA, 0.4 μM concentrations of all forward and reverse primers, 0.4 mM deoxynucleoside triphosphates, and 2.5 U Taq polymerase. Fragments were amplified on a PTC thermocycler (Bio-Rad, Hercules, CA) using a program of 95°C for 5 min; 40 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and a final extension of 72°C for 5 min. Fragments were separated by 1% agarose gel electrophoresis, stained with ethidium bromide, and visualized by UV light.
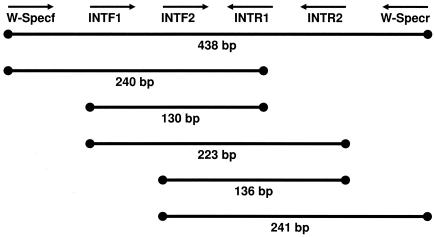
PCR was attempted using a variety of published (14, 28) and unpublished (Table 2) primer sets designed to specifically amplify portions of the Wolbachia 16S rRNA gene. Due to the wide range of DNA template quality in our samples, different primer combinations were used to amplify fragments ranging from approximately 130 bp to 900 bp. From degraded specimens, the amplification success rate for small fragments (<200 bp) was much greater than that for larger fragments. Primer sequences and amplified fragment arrangements are listed in Table 2 and Fig. 1.
TABLE 2.
Wolbachia-specific screening primers used in this study
| Primer | Sequence (5′-3′) | Reference |
|---|---|---|
| 99F | TTG TAG CCT GCT ATG GTA TAA CT | 14 |
| 994R | GAA TAG GTA TGA TTT TCA TGT | 14 |
| WSpecF | CAT ACC TAT TCG AAG GGA TAG | 28 |
| WSpecR | AGC TTC GAG TGA AAC CAA TTC | 28 |
| INTF1 | ACC CTC ATC CTT AGT TGC CAT | This study |
| INTR1 | TGT AGC ACG TGT GTA GCC CAC T | This study |
| INTF2 | AGT CAT CAT GGC CTT TAT GGA | This study |
| INTR2 | TCA TGT ACT CGA GTT GCA GAG T | This study |
FIG. 1.
Amplification schematic and approximate fragment sizes of WSpec and INT Wolbachia-specific 16S primers.
Sequencing.
While PCR screening was conducted with multiple specimens per species, sequences were obtained from a single positive specimen of each species. Amplified Wolbachia fragments were separated by 1% agarose gel electrophoresis, purified using QIAGEN MinElute columns (QIAGEN), and directly sequenced in both directions using an ABI Prism 3100 DNA sequencer (Applied Biosystems, Foster City, CA). BioEdit (6) software was used to manually edit sequences.
Phylogenetic analysis.
The GenBank database was searched for homologous sequences using the Basic Local Alignment Search Tool (BLAST). Retrieved sequences were aligned with manual correction using BioEdit. Maximum parsimony phylogenetic analyses were conducted using MEGA v. 2.1 (10). Tree support was evaluated by bootstrapping with 500 replications.
Nucleotide sequence accession numbers.
The sequences determined in this study were deposited in the GenBank database under accession numbers DQ399339 to DQ399349 and DQ400573.
RESULTS
In total, we assayed 39 Cimicidae species (spanning 16 genera and all 6 recognized subfamilies) (24) and 2 Polyctenidae species for Wolbachia infections (Table 1). The Wolbachia screening was attempted initially by specific amplification of an approximately 900-bp fragment of the Wolbachia 16S rRNA gene using primers 99F and 994R (14). Amplification of this fragment from recently collected wild specimens (A. constrictus and C. lectularius) generally succeeded but, with the exception of C. adjunctus, it was not successful when attempted with museum specimens. Amplification of an approximately 440-bp fragment from the same gene using primers WSpecF and WSpecR (28) produced similar results. We therefore attempted PCR using a set of internal primers (INTF1, INTF2, INTR1, INTR2) within the WSpec amplicon (Fig. 1; Table 2). These primers amplify overlapping fragments ranging from approximately 130 to 240 bp and were designed to be able to sequence the entire WSpec fragment in overlapping amplicons. Because the frequency of contaminant amplification increases with decreasing amplicon size, the 3′ base in each of the short primers was positioned at a synapomorphic site in Wolbachia relative to the common strains of background bacteria—Rickettsia spp., Ehrlichia spp., Anaplasma spp., and Cowdria spp.—making the primers Wolbachia specific. Not all primer combinations amplified and/or sequenced every specimen, likely due to degraded DNA and/or mutations in the primer binding sites. However, we were able to successfully amplify and confirm by sequencing at least one Wolbachia-specific fragment from nine different cimicid species and one polyctenid species (Table 1). Sequences were deposited in the GenBank database. The INTF2-INTR2 primer combination was used for initial screening because it produced a small amplicon and amplified the most consistently. Initial results (not shown) indicated that if the primer pair INTF2-INTR2 did not amplify the expected ∼130-bp fragment, other primer combinations never amplified any fragments. Thus, other primer combinations were not tested in later assays if the INTF2-INTR2 PCR failed.
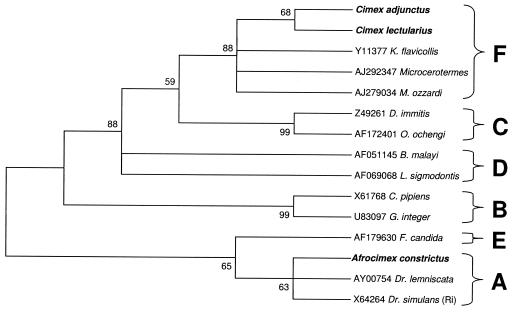
We were able to amplify the entire ∼900-bp 99F-994R fragment from infections of C. lectularius, Cimex adjunctus, and A. constrictus. The C. lectularius sequence was identical to that previously reported (18). Based on an analysis of 809 bp of the 99F-994R alignment, maximum parsimony analysis supported the inclusion of C. lectularius and C. adjunctus infections within the F supergroup (bootstrap support, 88%), similar to previously described results for C. lectularius Wolbachia infection (18). Analyses indicate inclusion of the A. constrictus infection in the A supergroup with weaker support (63%) (Fig. 2).
FIG. 2.
Maximum parsimony phylogenetic analysis of an 809-bp alignment of Wolbachia 16S sequences (99F-994R). Numbers at nodes indicate bootstrap support values (500 replicates). Taxon names are host species. Alphanumeric codes are GenBank accession numbers. Taxa in boldface type indicate cimicid species. Letters represent Wolbachia supergroup designations. The tree is unrooted but is presented as midpoint rooted for clarity.
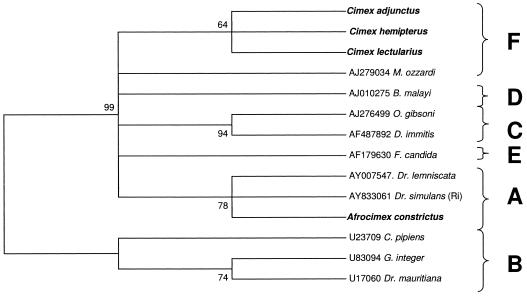
We were able to amplify the WSpec fragment from the infection of Cimex hemipterus by concatenating two internal amplified fragments (Table 1). We also were able to directly amplify the WSpec sequence from the A. constrictus, C. lectularius, and C. adjunctus infections. Based an analysis of a 418-bp WSpec alignment, the C. hemipterus infection was included in the F supergroup along with C. lectularius and C. adjunctus. Bootstrap support for this placement was weaker (64%) due to the smaller size of the nucleotide sequence. The A. constrictus infection was once again included in the A supergroup with moderate support (78%) (Fig. 3).
FIG. 3.
Maximum parsimony phylogenetic analysis of a 418-bp alignment of Wolbachia 16S sequences (WSpecF-WSpecR). Numbers at nodes indicate bootstrap support values (500 replicates). Taxon names are host species. Alphanumeric codes are GenBank accession numbers. Taxa in boldface type indicate cimicid species. Letters represent Wolbachia supergroup designations. The tree is unrooted but is presented as midpoint rooted for clarity.
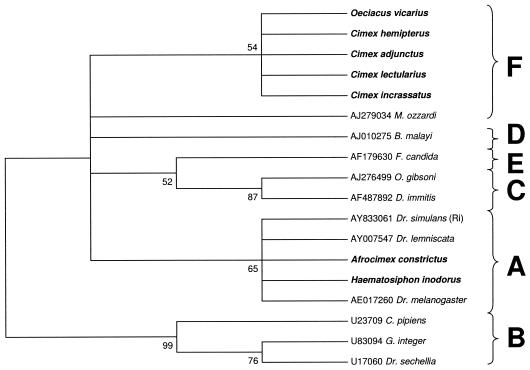
We were not able to amplify either the 99F-994R fragment or the entire WSpec fragment from our other specimens. We were, however, able to amplify smaller diagnostic fragments ranging from approximately 130 to 240 bp using various combinations of the internal INT primers. Maximum parsimony analysis of a 241-bp fragment amplified using primer pair INTF2-WSpecR supports the inclusion of Cimex incrassatus and Oeciacus vicarius in the F supergroup with weak support (54%) due to the small size of the sequence, confirming a previous identification of F Wolbachia in O. vicarius (18). The infection identified in Haematosiphon inodorus was placed in the A supergroup along with A. constrictus with relatively weak support due to the small size of the sequence (65%) (Fig. 4).
FIG. 4.
Maximum parsimony phylogenetic analysis of a 241-bp alignment of Wolbachia 16S sequences (INT2F-WSpecR). Numbers at nodes indicate bootstrap support values (500 replicates). Taxon names are host species. Alphanumeric codes are GenBank accession numbers. Taxa in boldface type indicate cimicid species. Letters represent Wolbachia supergroup designations. The tree is unrooted but is presented as midpoint rooted for clarity.
We were able to amplify, and confirm by sequencing, diagnostic Wolbachia fragments from the cimicids Cimex columbarius and Psitticimex uritui and from the polyctenid Hesperoctenes fumarius (Table 3), but we did not obtain enough sequence information to phylogenetically place these infections into a supergroup.
TABLE 3.
Wolbachia prevalence and supergroup designations as determined by PCR amplification and sequencing of diagnostic 16S rRNA gene fragments in selected Cimicidae and Polyctenidaea
| Species | Result with:
|
Wolbachia supergroup | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 99F, 994R | WSpecF, WSpecR | INT1F, INT1R | INT2F, INT2R | INT1F, INT2R | WSpecF, INT1R | WSpecF, INT2R | INT2F, WSpecR | ||
| Afrocimicinae | |||||||||
| Afrocimex constrictus | +* | +* | A | ||||||
| Cacodminae | |||||||||
| Aphrania vishnou | − | ||||||||
| Cacodmus vicinus | − | ||||||||
| Leptocimex boueti | − | − | |||||||
| Leptocimex duplicatus | − | ||||||||
| Loxapsis malayensis | − | ||||||||
| Stricticimex transversus | − | ||||||||
| Cimicinae | |||||||||
| Cimex adjunctus | +* | +* | + | + | − | + | + | F | |
| Cimex antennatus | − | − | − | ||||||
| Cimex brevis | − | − | − | ||||||
| Cimex columbarius | +* | − | − | ? | |||||
| Cimex hemipterus | + | +* | +* | F | |||||
| Cimex incrassatus | − | − | − | + | − | +* | F | ||
| Cimex insuetus | − | − | − | ||||||
| Cimex latipennis | − | − | |||||||
| Cimex lectularius | +* | +* | + | +* | + | + | F | ||
| Cimex pilosellus | − | − | − | − | − | − | − | ||
| Cimex pipistrellis | − | − | |||||||
| Cimex stadleri | − | − | |||||||
| Oeciacus hirudinis | − | − | |||||||
| Oeciacus vicarius | − | − | − | + | + | +* | F | ||
| Paracimex borneensis | − | ||||||||
| Paracimex caledoniae | − | ||||||||
| Paracimex capitatus | − | ||||||||
| Paracimex gerdheinrichi | − | ||||||||
| Paracimex inflatus | − | ||||||||
| Paracimex reductus | − | ||||||||
| Paracimex setosus | − | ||||||||
| Haematosiphoninae | |||||||||
| Haematosiphon inodorus | − | − | − | + | − | +* | A | ||
| Hesperocimex cochimiensis | − | ||||||||
| Hesperocimex coloradensis | − | − | − | − | − | − | − | − | |
| Hesperocimex sonorensis | − | ||||||||
| Ornithocoris furnarii | − | ||||||||
| Ornithocoris pallidus | − | − | − | − | − | − | − | ||
| Ornithocoris toledoi | − | ||||||||
| Psitticimex uritui | − | − | + | + | +* | − | − | + | ? |
| Latrocimicinae | |||||||||
| Latrocimex sp. | − | ||||||||
| Primicimicinae | |||||||||
| Bucicimex chilensis | − | − | |||||||
| Primicimex cavernis | − | − | |||||||
| Polyctenidae: Hesperocteninae | |||||||||
| Hesperoctenes eumops | − | − | |||||||
| Hesperoctenes fumarius | − | − | + | + | +* | − | − | + | ? |
*, sequence obtained; +, Wolbachia positive; −, Wolbachia negative; ?, unknown supergroup; blank cells, nontested primer combinations. Boldface type indicates Wolbachia-positive species. Wolbachia supergroup designations are as denoted in Results.
DISCUSSION
Our survey results suggest that Wolbachia infections may be common in the Cimicidae. In this preliminary screen of 39 cimicid species, we identified nine infections, seven of which were newly described. Additionally, we observed Wolbachia infection in one of the Polyctenidae (sister family to the Cimicidae). We demonstrated that at least two different Wolbachia supergroups infect cimicids. We reconfirmed the presence of F supergroup Wolbachia in C. lectularius and O. vicarius and identified related F supergroup infections in the Cimex congenerics C. hemipterus, C. adjunctus, and C. incrassatus. These results suggest that F supergroup infections may be common in the subfamily Cimicinae. We were able to place the Wolbachia infections of A. constrictus and H. inodorus into the A supergroup. Wolbachia A supergroup infections are commonly described and infect diverse arthropods (11, 21, 27, 28). It remains to be seen, however, how prevalent Wolbachia supergroup A infections are in Cimicidae. We did not obtain sufficient sequence information to confidently phylogenetically place the Wolbachia infections of C. columbarius, Psitticimex uritui, and Hesperoctenes fumarius. Definitive phylogenetic placement of these infections is not possible without additional sequence data.
The observation of multiple F supergroup infections among the subfamily Cimicinae is very striking (Table 3). Monophyletic F infections were observed for two genera (Cimex and Oeciacus), suggesting that in this subfamily, Wolbachia was introduced once and has diverged dependently along with the insect hosts. In contrast, A supergroup infections were detected in two widely divergent subfamilies, suggesting multiple introductions of A infections into the Cimicidae. Future surveys to detail the distribution of F and A infections among cimicid and polyctenid species are clearly warranted.
The results presented in this initial survey are almost certainly an underestimate of Wolbachia prevalence in cimicids. The failure to detect Wolbachia DNA in many species may have been due to true lack of infection, sampling bias due to small sample sizes or, most likely, poor template quality in insufficiently preserved specimens. Many specimens, especially those from the Usinger Collection, were stored without temperature control or ethanol changes for over 40 years. Our results are thus preliminary and should be used to guide future survey efforts using fresh wild-caught material. However, even with poorly preserved material, we observed an infection rate in cimicids of over 23% (9 of 39 species), comparable to other estimates of Wolbachia prevalence in arthropod taxa (28).
We have shown in this study that museum specimens can provide a valuable resource for molecular surveys of Wolbachia infections, similar to results obtained for other bacterial species. Despite DNA degradation, we were able to amplify diagnostic fragments from ethanol-preserved specimens up to 48 years old. While not all of these fragments were long enough to be phylogenetically useful, sequencing confirmed that they were all diagnostic for Wolbachia. Due to the small sizes of the PCR amplicons, the screening primers listed in this report work well for specimens with less-than-optimum DNA quality and should be useful for other researchers conducting Wolbachia surveys.
Acknowledgments
This work was supported by the Johns Hopkins Malaria Research Institute and NSF FIBR grant EF-0328363.
We thank Douglas Norris, Rebekah Kent, Nixon Wilson, Carl Dick, Will Reeves, and Klaus Reinhardt for providing cimicid and polyctenid specimens from their field sites and private collections for use in our study. We are especially grateful to Cheryl Barr for generously allowing us to assay specimens from the Usinger Cimicid Collection (Essig Museum, University of California, Berkeley). We thank Cheryle O'Donnell for advice concerning nondestructive DNA extraction, Catherine Westbrook for advice with primer selection and screening, and John H. Werren and three anonymous reviewers for comments that significantly improved the manuscript.
REFERENCES
- 1.Arkwright, J. A., E. E. Atkin, and A. Bacot. 1921. An hereditary Rickettsia-like parasite of the bed bug (Cimex lectularius). Parasitology 13:27-36. [Google Scholar]
- 2.Barns, I., J. Holton, D. Vaira, M. Spigelman, and M. G. Thomas. 2000. An assessment of the long-term preservation of the DNA of a bacterial pathogen in ethanol-preserved archival material. J. Pathol. 192:554-559. [DOI] [PubMed] [Google Scholar]
- 3.Beard, C. B., S. L. O'Neill, R. B. Tesh, F. F. Richards, and S. Aksoy. 1993. Modification of arthropod vector competence via symbiotic bacteria. Parasitol. Today 9:179-183. [DOI] [PubMed] [Google Scholar]
- 4.Bordenstein, S., and R. B. Rosengaus. 2005. Discovery of a novel Wolbachia supergroup in Isoptera. Curr. Microbiol. 51:393-398. [DOI] [PubMed] [Google Scholar]
- 5.Chang, K. P., and A. J. Musgrave. 1973. Morphology, histochemistry, and ultrastructure of mycetome and its rickettsial symbiotes in Cimex lectularius L. Can. J. Microbiol. 19:1075-1081. [DOI] [PubMed] [Google Scholar]
- 6.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 7.Hubbard, M. J., A. S. Baker, and K. J. Cann. 1998. Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixododae) since the 19th century, assessed by PCR. Med. Vet. Entomol. 12:89-97. [DOI] [PubMed] [Google Scholar]
- 8.Hypsa, V., and S. Aksoy. 1997. Phylogenetic characterization of two transovarially transmitted endosymbionts of the bedbug Cimex lectularius (Heteroptera: Cimicidae). Insect Mol. Biol. 6:301-304. [DOI] [PubMed] [Google Scholar]
- 9.Konomi, N., E. Lebwohl, K. Mowbray, I. Tattersall, and D. Zhang. 2002. Detection of mycobacterial DNA in Andean mummies. J. Clin. Microbiol. 40:4738-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 11.Lo, N., M. Casiraghi, E. Salati, C. Bazzocchi, and C. Bandi. 2002. How many Wolbachia supergroups exist? Mol. Biol. Evol. 19:341-346. [DOI] [PubMed] [Google Scholar]
- 12.Marshall W. F., III, S. R. Telford III, P. N. Rys, B. J. Rutledge, D. Mathiesen, S. E. Malawista, A. Spielman, and D. H. Persing. 1994. Detection of Borrelia burgdorferi DNA in museum specimens of Peromyscus leucopus. J. Infect. Dis. 170:1027-1032. [DOI] [PubMed] [Google Scholar]
- 13.Matuschka, F. R., A. Ohlenbusch, H. Eiffert, D. Richter, and A. Spielman. 1996. Characteristics of Lyme disease spirochetes in archived European ticks. J. Infect. Dis. 174:424-426. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill, S. L., R. Giordano, A. M. E. Colbert, T. L. Karr, and H. M. Robertson. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persing, D. H., S. R. Telford III, P. N. Rys, D. E. Dodge, T. J. White, S. E. Malawista, and A. Spielman. 1990. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science 249:1420-1423. [DOI] [PubMed] [Google Scholar]
- 16.Rasgon, J. L., and T. W. Scott. 2003. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics 165:2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasgon, J. L., L. M. Styer, and T. W. Scott. 2003. Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J. Med. Entomol. 40:125-132. [DOI] [PubMed] [Google Scholar]
- 18.Rasgon, J. L., and T. W. Scott. 2004. Phylogenetic characterization of Wolbachia symbionts infecting Cimex lectularius L. and Oeciacus vicarius Horvath (Hemiptera: Cimicidae). J. Med. Entomol. 41:175-178. [DOI] [PubMed] [Google Scholar]
- 19.Rowley, S. M., R. J. Raven, and E. A. McGraw. 2004. Wolbachia pipientis in Australian spiders. Curr. Microbiol. 49:208-214. [DOI] [PubMed] [Google Scholar]
- 20.Sinkins, S. P. 2004. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 34:723-729. [DOI] [PubMed] [Google Scholar]
- 21.Stouthamer, R., J. A. J. Breeuwer, and G. D. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 22.Turelli, M., and A. A. Hoffmann. 1999. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol. Biol. 8:243-255. [DOI] [PubMed] [Google Scholar]
- 23.Ueshima, N. 1964. Experiments on reproductive isolation in Cimex lectularius and Cimex columbarius. Pan-Pac. Entomol. 40:47-53. [Google Scholar]
- 24.Usinger, R. L. 1966. Monograph of Cimicidae. Entomological Society of America, College Park, Md.
- 25.Vandekerckhove, T. T., S. Watteyne, A. Willems, J. G. Swings, J. Mertens, and M. Gillis. 1999. Phylogenetic analysis of the 16S rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for wolbachial taxonomy. FEMS Microbiol. Lett. 180:279-286. [DOI] [PubMed] [Google Scholar]
- 26.Wernegreen, J. J. 2002. Genome evolution in bacterial endosymbionts of insects. Nat. Rev. Genet. 3:850-861. [DOI] [PubMed] [Google Scholar]
- 27.Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. Biol. Sci. 261:55-63. [DOI] [PubMed] [Google Scholar]
- 28.Werren, J. H., and D. M. Windsor. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. Biol. Sci. 267:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi, Z., C. C. Khoo, and S. L. Dobson. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310:326-328. [DOI] [PubMed] [Google Scholar]
- 30.Zabalou, S., M. Riegler, M. Theodorakopoulou, C. Stauffer, C. Savakis, and K. Bourtzis. 2004. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 101:15042-15045. [DOI] [PMC free article] [PubMed] [Google Scholar]