Fig. 4. G418 increases recombinant GPx-1 protein (rGPx-1) but decreases rGPx-1 activity.
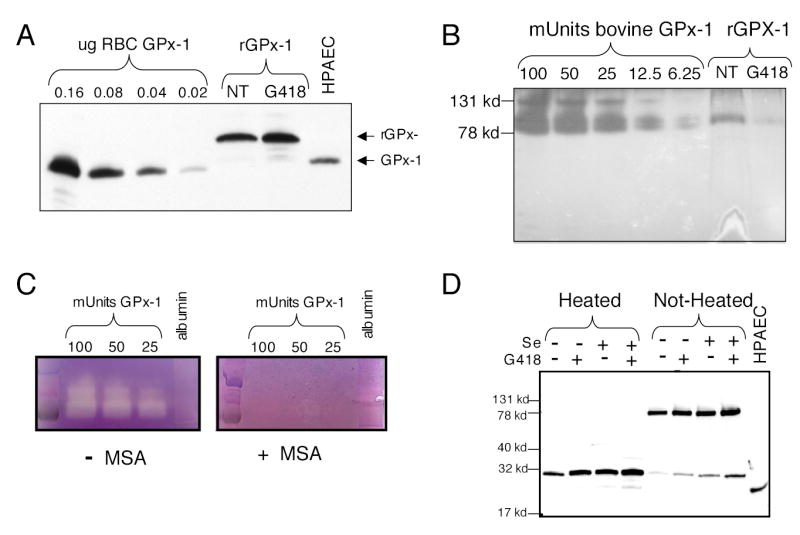
Recombinant protein was isolated from permanently transfected cells grown in media with 2% FCS with the addition of selenium (10ng/ml) and/or G418 (200 μg/ml). His-tagged rGPx-1 was isolated using Ni-magnetic beads. Samples were analyzed by Western blot with the mouse anti-human GPx-1 antibody and by in-gel enzyme activity assays. (A) Western blot of rGPx-1 isolated from cells treated with G418 and those not treated (NT). Various amounts of commercially available human GPx-1 were used as controls on the gel. HPAEC indicates samples from human pulmonary arterial endothelial cells. (B) Reversed image of an in-gel GPx-1 assay using the same rGPx-1 samples used for panel (A). Commercially available bovine GPx-1 was used as standards for the in-gel assay. Samples were run on SDS/PAGE gel without heat denaturation to preserve the active form of the enzyme. (C) The in-gel assay is specific for GPx-1 activity. MSA, a GPx-1 inhibitor, blocks the formation of the achromatic bands. Albumin (1 μg) was used as a non-specific protein. (D) Western blot or rGPx-1 from cells treated with G418 or selenium. Heat-denaturation reduces the rGPx-1 to the monomeric form. Most of the rGPx-1 is in the active tetrameric form (non-heated sample).
