Abstract
Acquisition of milk production capabilities by an ancestor of mammals is at the root of mammalian evolution. Milk casein micelles are a primary source of amino acids and calcium phosphate to neonates. To understand the role of κ-casein in lactation, we have created and characterized a null mouse strain (Csnk−/−) lacking this gene. The mutant κ-casein allele did not affect the expression of other milk proteins in Csnk−/− females. However, these females did not suckle their pups and failed to lactate because of destabilization of the micelles in the lumina of the mammary gland. Thus, κ-casein is essential for lactation and, consequently, for the successful completion of the process of reproduction in mammals. In view of the extreme structural conservation of the casein locus, as well as the phenotype of Csnk−/− females, we propose that the organization of a functional κ-casein gene would have been one of the critical events in the evolution of mammals. Further, κ-casein variants are known to affect the industrial properties of milk in dairy animals. Given the expenses and the time scale of such experiments in livestock species, it is desirable to model the intended genetic modifications in mice first. The mouse strain that we have created would be a useful model to study the effect of κ-casein variants on the properties of milk and/or milk products.
Keywords: casein locus, evolution, lactation, milk, micelle
Milk production is an essential component of the reproductive strategy of mammals. The development of a zygote into an organism is accompanied by the preparation of milk synthesis potential by the mammary gland. Perinatal survival in mammals is solely dependent on the availability of milk from the mother. Caseins account for >80% of the total milk proteins (1). These proteins exist as micelles, consisting of three to four phosphoproteins (i.e., the calcium-sensitive caseins: α-, β-, γ-, and δ-casein in mice) and a calcium-insensitive phosphoglycoprotein, κ-casein (2). In addition to functioning as the primary source of amino acids, one of the key functions of micelles is to sequester large amounts of calcium phosphate from the maternal diet or bodily stores and make it readily available to the newborn. Calcium-sensitive casein genes seem to have evolved from a primitive casein gene through gene duplication and exon shuffling (3); however, it appears that κ-casein is not related structurally and evolutionarily to these genes. Instead, it appears to be related to γ-fibrinogen (4, 5). Recently, it has also been speculated that both the calcium-sensitive and the calcium-insensitive casein gene belong to one gene family (6). The null mutation of the β-casein gene in mice (7) and that of αS1-casein in goats (8) do not show any significant effect on milk micelle formation and lactation. The lack of adverse effects on micelle formation in these mutants suggests functional redundancy amongst calcium-sensitive caseins. However, the absence of αS1-casein in goats does affect the rate of transport of caseins. The experimental evidence (9–12) tends to support various models of micelle structure (13, 14) that envisage the presence of most of the κ-casein on the outer surfaces of casein micelles, and the organization of micelles in vitro requires κ-casein (12, 13). It is not known whether κ-casein is essential for the assembly of the casein micelles in vivo and for milk production and/or secretion. Interestingly, all of the casein genes are arranged in a locus in all of the mammals examined thus far. Although the protein sequences of casein genes are very poorly conserved, the organization of this locus, including the gene structure, gene order, and the orientation, has been very well conserved (2). The functional and structural constraints defining this extreme conservation remain unclear. To address the role of κ-casein in lactation and the significance of the location of this gene in the neighborhood of calcium-sensitive casein genes across species, we have created and characterized a κ-casein null mouse strain.
Results
Generation of Csnk−/− Mice.
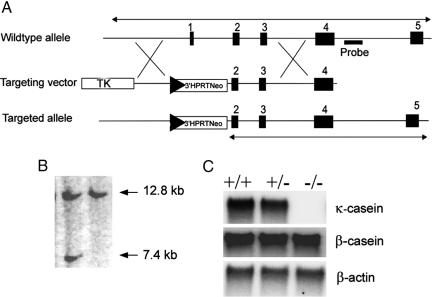
To introduce a null mutation in the κ-casein gene, we replaced a 3.3-kb fragment comprising exon 1 (untranslated) and 5′ upstream sequences with a cassette of neomycin selectable marker gene, a LoxP site, and 3′ part of the Hprt minigene in a 9.2-kb genomic DNA fragment from the casein locus (Fig. 1A). A LoxP site and partial Hprt gene sequences were placed in the targeting vector for the subsequent utilization of the manipulated ES cells for introduction of long-range modifications in the casein gene complex. This vector was used to mutate the κ-casein gene in embryonic stem cells (Fig. 1 A and B), and, subsequently, the mice carrying the mutant allele were derived from the manipulated embryonic stem cells. The heterozygous mice were mated to produce a mutant homozygote (Csnk−/−) mouse strain. The genotypes of the progeny from these matings were as per Mendelian segregation ratios.
Fig. 1.
Targeting of the κ-casein gene. (A) Upon targeting, exon 1 and 3 kb of upstream region are replaced with a cassette containing LoxP, 3′ Hprt gene sequences, and the neomycin gene. (B) Southern blot analysis of targeted ES cells showing a 7.4-kb BamHI fragment hybridizing to a probe external to the targeting vector. (C) Northern blot analysis of κ-casein gene expression in the mammary gland (postpartum day 0) showing lack of transcript in Csnk−/− mice and gene dosage effect in Csnk+/− mice in comparison with the wild-type mouse. β-Casein gene expression in the absence of κ-casein gene expression is not affected. Blots were reprobed with β-actin cDNA for normalization of total RNA loaded.
Csnk−/− Mice Fail to Suckle Their Pups.
To determine the effect of this mutation on fertility, lactation, casein gene expression, and the properties of milk, the wild-type (Csnk+/+), mutant heterozygote (Csnk+/−), and mutant homozygote (Csnk−/−) females were bred to the respective males. κ-Casein null mice were viable. The Csnk−/− females were fertile and carried the pregnancy to term. However, the pups from these mothers did not survive >8–10 h. Autopsy of the pups did not reveal any milk in their guts. Successful fostering of these pups to appropriate lactating females confirmed that Csnk−/− females did not suckle their pups. Efforts to milk these females after oxytocin injections yielded no milk but did yield a yellowish secretion in extremely low volumes. The Csnk−/− females were unable to suckle their pups because of failure of lactation.
κ-Casein Allele Did Not Affect the Expression of Other Milk Proteins.
Milk protein gene expression was analyzed by Northern blot analysis on the mammary gland tissues (postpartum day 0). The lack of κ-casein transcript in Northern blots from Csnk−/− females (Fig. 1C) confirmed that the allele that we constructed is indeed a null allele. The level of κ-casein mRNA in Csnk+/− mice was lower than that in Csnk+/+ mice, indicating gene dosage effect at this locus, as reported earlier in the case of the β-casein gene (7). It was possible that a null mutation in the κ-casein gene would affect the expression of the other casein genes because they are tightly linked. Northern blot experiments with β-casein cDNA probes did not reveal any significant differences among Csnk+/+, Csnk+/−, and Csnk−/− females (Fig. 1C). Similarly, the expression of other casein genes was also not affected by this mutation (data not shown). WAP (whey acidic protein) and α-lactalbumin genes lie outside the casein locus. The steady-state mRNA levels from these two genes in Csnk+/− and Csnk−/− females were comparable to that of the wild type (Fig. 6, which is published as supporting information on the PNAS web site). The total milk protein content did not differ significantly between Csnk+/+ (105.3 ± 1.37 mg·ml−1) and Csnk+/− (108.4 ± 2.86 mg·ml−1) mice. Thus, the mutant κ-casein allele did not affect the expression of other milk proteins in Csnk+/− and Csnk−/− females.
Calcium-Sensitive Caseins Were Secreted but Aggregated in the Alveolar Lumina of Csnk−/− Mammary Glands.
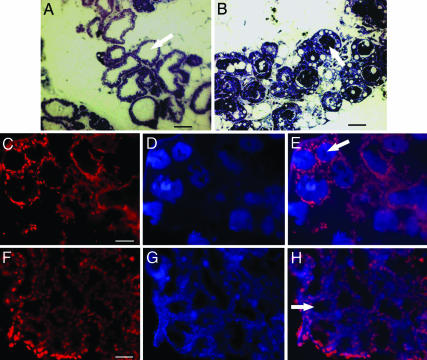
To evaluate whether the failure of lactation in Csnk−/− mice was due to gross developmental defects in the mammary gland or due to failure of milk formation and/or secretion, the anatomy of the mammary gland and its extent of distribution in Csnk−/− mice were examined. We also compared the average ratios of mammary gland weight to the whole-animal weight of Csnk−/− mice with that of Csnk+/− and Csnk+/+ mice. No significant difference was observed either in the gross anatomy or the weight ratios of Csnk−/− mice compared with that of Csnk+/− and Csnk+/+ mice. This finding was further confirmed by the histological examination of mammary gland tissue. The semithick sections of the mammary gland showed normal mammary gland secretory epithelium arranged in alveoli in Csnk−/− females (Fig. 2B). However, unlike in Csnk+/+ mice (Fig. 2A), the alveolar lumina in Csnk−/− females showed darkly stained aggregates of proteins, which were speculated to be the other caseins. To ascertain the nature of these proteinaceous aggregates in the lumina, the sections were immunostained with Alexa Fluor 350-labeled antibodies against mouse β-casein (Fig. 2 D and G) and the mouse total casein (data not shown). The positive staining of these aggregates in Csnk−/− females (Fig. 2D) confirmed the presence of calcium-sensitive caseins in them. Thus, in the absence of κ-casein, the remaining caseins were being secreted into the lumina but were coagulated and retained in the lumina. These observations showed that the calcium-sensitive caseins were produced and also secreted into the alveolar lumina of the mammary gland but could not be ejected out of the mammary gland.
Fig. 2.
Calcium-sensitive caseins aggregate in the Csnk−/− mammary gland (postpartum day 0). (A) Toludine blue staining of semithick plastic sections of wild-type mammary gland shows well formed alveoli with clear lumina (arrow). (B) In Csnk−/− mice, alveolar lumina (arrow) are blocked with proteinaceous precipitates. (D and G) Sections of Csnk−/− and wild-type mammary gland, respectively, stained with Alexa Fluor 350-labeled antibodies against mouse β-casein protein. (C and F) Staining of nuclei with propidium iodide (PI). E and H are overlaps of Alexa Fluor 350 and PI staining. These observations confirm that aggregates in Csnk−/− lumina (arrow) are other caseins. (Scale bars, 10 μm.)
Calcium-Sensitive Caseins Aggregate in Midpregnancy in Csnk−/− Mammary Glands.
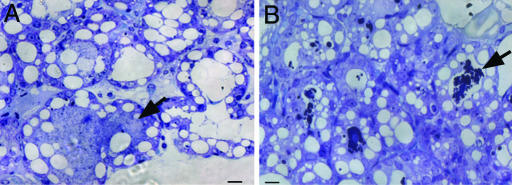
The casein gene expression starts during midpregnancy. To examine whether the calcium-sensitive caseins were coagulating in the alveolar lumina during pregnancy, we analyzed the mammary glands of wild-type and Csnk−/− females from 16.5 days postcoitum (dpc) onward. The semithick sections of the wild-type mammary glands revealed no aggregates in the lumina (Fig. 3A). However, in Csnk−/− mice, the aggregates of calcium-sensitive caseins were already present (Fig. 3B). Thus, the remaining calcium-sensitive caseins were synthesized and secreted into the alveolar lumina from the epithelial cells, which formed the proteinaceous aggregates in Csnk−/− mammary glands at least from 16.5 dpc onward.
Fig. 3.
Toludine blue staining of mammary gland sections of wild-type and Csnk−/− mice at midpregnancy (16.5 dpc). (A) Alveolar lumina (arrow) of the wild type lack precipitates. (B) Precipitates have started appearing in Csnk−/− alveolar lumina. (Scale bars, 5 μm.)
Calcium-Sensitive Caseins Initiate Formation of Micelle-Like Structures in Vivo in Csnk−/− Milk.
To determine whether the calcium-sensitive caseins in the alveolar lumina of Csnk−/− mice were secreted as discrete proteins or whether they were secreted in any organized form, ultrathin sections of the mammary gland were analyzed by immunogold staining and electron microscopy (Fig. 4). Ultrastructural analysis of mammary glands of both Csnk+/+ and Csnk−/− mice revealed no differences in gross structure. Acinar cells had a normal endoplasmic reticulum (ER) with no evidence of dilation, secretory vesicles with multiple granules on the apical side, luminar membranes with microvilli, normal mitochondria, and nucleus on the basal side of the cells. Only the lumina of Csnk−/− mice were full of aggregates, which stained positive for caseins (Fig. 4B). The presence of discrete (at times, multiple) particles in the secretory vesicles in the mammary gland epithelium of Csnk−/− mice suggested that the calcium-sensitive caseins were assembling into discrete micelle-like structures even in the absence of κ-casein.
Fig. 4.
Calcium-sensitive caseins are organized into micelle-like structures in the absence of κ-casein. (A) Immunogold staining of the wild-type mammary gland (postpartum day 0). (B) Immunogold staining of a Csnk−/− gland (postpartum day 0). Multiple micellar like structures are present in the secretory vesicles of Csnk−/− glands as in that of the wild type. However, they are seen coalescing after secretion in Csnk−/− mice, unlike in the wild type. Anti-mouse casein serum raised in rabbits was used for immunogold staining. L, lumen; M, micelles; SV, secretory vesicles; AM, apical membrane. (Scale bars, 0.1 μm.)
κ-Casein Concentration Affects Topology of Casein Micelles.
To determine the effect of κ-casein concentration on the micelle topology, milk samples were defatted and analyzed by atomic force microscopy (AFM). AFM analysis of the topology of milk showed discrete micelles in Csnk+/+ milk (Fig. 5A), unlike Csnk+/− milk, which revealed coalescence of the micelles (Fig. 5B).
Fig. 5.
Casein micelles show coalescence in Csnk+/− milk. (A) AFM examination of Csnk+/+ micelles; scan size is 2.5 μm. (B) Some of the Csnk+/− micelles coalesce to form larger, irregularly shaped structures.
Discussion
Our experiments show that the κ-casein gene-deficient mice developed normally. However, the females failed to lactate and suckle their pups, which led to perinatal death. In the Csnk−/− mouse, the mammary gland is morphologically developed and, physiologically, it secretes caseins into the alveolar lumina. We analyzed the expression of casein genes, the early markers for differentiation of mammary secretory epithelium, and that of WAP and α-lactalbumin genes, the late markers (15). The expression of these marker genes in Csnk−/− mice is not different from that of the wild type from 12.5 dpc onward (data not shown) or at postpartum day 0 (Fig. 6). Thus, the κ-casein gene is neither essential for the development of the mammary gland nor for the normal differentiation of mammary secretory epithelium. The presence of multiple particles in the secretory vesicles and in the alveolar lumina as revealed by electron microscopy suggests that κ-casein is not essential for initiation of organization of calcium-sensitive caseins into micelle-like structures or for their secretion into the lumina. The micelle-like structures are formed in the secretory vesicles and emptied into the lumina of κ-casein-deficient mammary glands. Trafficking of the proteins seems to be unaffected; the proteins are being synthesized in the ER stacks in the basal part of the cell, and the granules are seen in the vesicles in the apicular part of the cell. Moreover, the appearance of the casein aggregates in Csnk−/− mice is concurrent with the secretion of caseins into the wild-type lumina. However, we have not looked into the rate of secretion of the micelle-like structures from the epithelial cells. Thus, at present it is not known whether the κ-casein null mutation has any effect on the rate of trafficking of the remaining proteins. Our earlier studies showed that in the β-casein-deficient mouse mammary gland, the formation of casein micelles and the time course of secretion were not affected (7, 16). However, a null mutation of goat αS1-casein is known to slow down the rate of transport of the other caseins (8). A recent in vitro study has shown that in the absence of αS1-casein, κ-casein aggregates to form amyloid bodies (17). It may be possible that αS1-casein aids in the assembly of caseins in the ER and facilitates their transport by preventing aggregation of κ-casein (18).
The failure of lactation in Csnk−/− mice is due to blockage of the alveolar lumen and the mammary gland ducts by the protein aggregates. It is possible that the protein aggregates induced ER stress, leading to translational and transcriptional attenuation (19). However, it may be noted that there is no evidence for retention of the caseins in the endoplasmic reticula in our electron micrographs. Further, we performed analyses for the presence of possible ER stress-induced splicing of XBP1 (X box-binding protein 1) transcript by RT-PCR in the postpartum day 0 mammary glands (Fig. 7, which is published as supporting information on the PNAS web site). We did not find any evidence in support of ER stress in these glands.
Various models have been proposed to explain the structure of casein micelles (20–25). All of these models envisage κ-casein as an integral component of micelle structure. The micelle size is determined by the κ-casein ratio to the calcium-sensitive caseins (7, 26); the higher ratio of κ-casein to calcium-sensitive caseins results in smaller micelles. These observations are consistent with the surface localization of κ-casein that is postulated by most of the above models. Our AFM studies demonstrate that the decrease in the κ-casein concentration led to coalescence of micelles in Csnk+/− milk. Comparatively low levels of κ-casein would probably leave surface areas free of κ-casein in some of the micelles, thus allowing the interaction of calcium-sensitive caseins across such micelles. The appearance of multiple micellar structures in the secretory vesicles of Csnk−/− mammary epithelium would suggest that κ-casein is not required for the aggregation of the calcium-sensitive caseins into micelle-like structures. However, we cannot conclude whether the structural organization of the calcium-sensitive caseins in the micelle-like particles is similar to that of the wild-type micelles. It has been suggested that the organization of micelles continues even after their secretion into the lumina (27). It is very likely that the absence of κ-casein severely interrupts this process of micelle reorganization in the alveolar lumen and leads to destabilization of the secreted particles, culminating in aggregation of the calcium-sensitive caseins in the alveolar lumina.
Sequences of the casein genes are poorly conserved during evolution (2, 3, 28). Of all of the caseins, κ-casein is the most conserved in terms of its molecular architecture. Not all calcium-sensitive caseins are found in all mammals, but at least one of these caseins is present in all milks. On the other hand, κ-casein or κ-casein-like protein is invariably found in all of mammals examined so far, including the marsupials (2, 13). Notwithstanding the slowing down effect of the αS1-casein null mutation on casein transport in goats, lactation is sustained in these animals (8). In β-casein-deficient mice, neither casein transport nor lactation is affected (7, 16). Thus, there is a redundancy among the various calcium-sensitive caseins that augments survival benefits. Contrary to this observation, the present study demonstrates that the loss of κ-casein in the mouse leads to failure of lactation and, as a result, interruption in reproduction.
Despite poor conservation of the casein gene sequences, their organization, gene order, and orientation seem to have been highly conserved, at least for the past 80–100 million years (2, 28). In all of the modern-day mammals in which organization of this locus has been studied, the κ-casein gene is invariably linked with the calcium-sensitive casein genes. The functional and structural constraints leading to highly conserved gene organization are not yet understood. The κ-casein null mouse phenotype does suggest that one of the imperatives for retaining κ-casein in the “casein gene expression neighborhood” may be the requirement of extremely well synchronized temporal regulation of this gene along with other caseins. We observed proteinaceous aggregates of the calcium-sensitive caseins in the mammary glands of null mice at least from 16.5 dpc onward, which means that κ-casein needs to be available for the stabilization of micelles during midpregnancy and therefore, for successful lactation, either before or concurrent to the synthesis of calcium-sensitive caseins. We suggest that such a stringent requirement for temporal gene regulation at this locus has been achieved through extreme conservation of organization of the casein locus during mammalian divergence.
It is generally believed that the formation of a true mammary gland with complex alveolar structures, as it exists in modern mammals, is a late event compared with the secretion of “milk” by an ancestor of mammals. Assuming that only a fraction of the dietary calcium phosphate requirement of the newborn of an ancestor of present-day mammals was initially met through “milk” secretion, primitive casein(s) would have had a comparatively smaller number of phosphorylation sites. In such a scenario, one can envisage that secretion of such caseins did not require a stabilizing protein like κ-casein. Subsequent evolution of the mammary gland with alveolar structures and incorporation of multiple phosphorylation sites into the primitive casein molecule and the duplication of casein genes would have resulted into multiple calcium-sensitive caseins with an increased calcium phosphate sequestering capacity. These events would have paved the way for divergence of mammals into various species with well developed skeletal systems and predatory powers. However, before these changes could happen, stability of this complex casein micelle needed to be addressed, and, hence, organization of a functional κ-casein gene would have been an absolute requirement. It has been suggested that the κ-casein gene would have arisen through exon shuffling and deletion of introns from a γ-fibrinogen-like gene (29, 30). Alternatively, the κ-casein gene would have evolved from a primitive gene within the casein locus (6). Given that only the C terminus of κ-casein has homology with γ-fibrinogen, it might be possible that a primitive casein gene in this locus acquired the exonic sequences from a γ-fibrinogen-like gene. However, one cannot rule out convergent evolution (29), as has also been recently suggested for the C terminus of β-casein (31). It may be noted that irrespective of the mechanism(s) involved, it would appear that once a functional κ-casein gene was opted as a part of the casein locus in a primitive mammal, the order and placement of this gene remained unchanged during the mammalian evolutionary history.
κ-Casein variants are known to affect the industrial properties of milk in dairy animals (32). Genetic manipulation of cattle with an objective to alter the milk properties is being attempted successfully (33). Given the expenses and the time scale of such experiments in livestock species, it is desirable to model the intended genetic changes in the mouse first. The mouse strain that we have created would be a useful model to the study the effect of κ-casein variants on the properties of milk and milk products.
We have shown that in contrast to the calcium-sensitive caseins, κ-casein is indispensable for lactation and, consequently, for reproduction in mammals. The failure of lactation results from destabilization of casein micelles in the alveolar lumina. We propose that recruitment of a primitive κ-casein molecule to stabilize the casein micelles, achieved through coopting a functional κ-casein-like gene in the casein locus, would have been a significant event in mammalian evolution. Analysis of this locus in the monotremes should further strengthen this proposition. ESTs have been identified for three calcium-sensitive casein genes in the platypus, but there is no evidence as yet for the presence of a κ-casein-like protein (J. A. Sharp, personal communication). Further experiments aimed at the expression of the κ-casein gene in the Csnk−/− mouse at various time points during pregnancy by a conditional transgene could shed light on the requirement of a stringent temporal coregulation of calcium-sensitive and calcium-insensitive casein genes and, hence, would help to reveal one of the possible functional constraints for the extreme conservation of structural organization of this locus in mammals.
Materials and Methods
Construction of the κ-Casein Gene Targeting Vector.
To construct the κ-casein gene targeting vector, a 3.3-kb region of this gene including exon 1 and the 5′ upstream region from a 9.2-kb isogenic genomic fragment from this locus was replaced with a NotI–BamHI fragment containing a LoxP site, partial Hprt gene sequences, and a neomycin marker gene from pMC1Neo (Stratagene). The resultant vector had 2.0- and 3.9-kb arms of homology at the 5′ and 3′ ends of the marker cassette, respectively. To enrich for targeting events, a HSV tk gene was placed before the 5′ end of homologous sequences (Fig. 1A).
Generation of κ-Casein Null Mice.
Forty micrograms of linearized targeting vector DNA was electroporated into R1.9 (34), a subclone of the R1 ES cell line (35). The cells were selected with geneticin (0.25 mg·ml−1) and ganciclovir (2 μM). Genomic DNA from ES cell clones was analyzed by Southern hybridization using a 550-bp genomic probe external to the 3′ sequences included in the targeting vector. Targeted clones were injected into 3.5-dpc C57/BL6 blastocysts, and the latter were transferred into the uteri of CD1 pseudopregnant females. To obtain the germ-line transmission of the mutant allele, chimeric males were mated to CD1 females.
RNA Isolation and Northern Blot Analysis.
Mammary gland tissues were collected from female mice on the day of delivery and were immediately frozen in liquid nitrogen and stored at −80°C until further use. Total cellular RNA was isolated by using TRIzol reagent (Invitrogen). RNA was size-fractionated in 1% agarose gel containing 2.2 M formaldehyde and blotted on to Hybond N+ membrane in 10× standard saline phosphate/EDTA . β-, α-, γ-, κ-Casein, WAP, and α-lactalbumin cDNAs were radiolabeled by random priming. Hybridization was performed overnight at 68°C in 0.5 M phosphate buffer/7% SDS/1 mM EDTA, and the membranes were washed twice at 68°C for 15 min each in 40 mM phosphate buffer/1% SDS/1 mM EDTA. The membranes were stripped and rehybridized with labeled β-actin cDNA for loading control.
Milk Protein Assay.
Milk was defatted and diluted 1:1,000 with water, and total protein was assayed by using Bradford’s reagent (Bio-Rad) per the manufacturer’s instructions with BSA as a standard.
Transmission Electron Microscopy.
The mammary glands of the wild-type, heterozygous, and homozygous mice were dissected postpartum (day 0) and fixed in 2.5% glutaraldehyde in phosphate buffer. Briefly, the glands were postfixed in 1% osmium tetraoxide, followed by dehydration, infiltration in resin (araldite–dodecenyl succinic anhydride mixture), and embedding. The blocks were cured at 60°C for 3 days and sectioned. Semithick sections for each sample were stained with toludine blue to study the gross histology; micrographs were recorded with a Zeiss Axioplan microscope with a digital camera. The ultrathin sections were stained with uranyl acetate and lead citrate (36) and scanned with a JEOL 100CX transmission electron microscope at 80 kV using a 20-μm aperture (37). For immunogold microscopy, the glands were fixed in 4% paraformaldehyde for 15 min, dehydrated, infiltrated, and embedded in LR gold resin (London Resin, Basingstoke, U.K.). The blocks were cured at 45°C overnight and sectioned. Immunostaining was performed with anti-mouse casein serum raised in rabbits (a kind gift from A. Kolb, Hannah Research Institute, Ayr, Scotland) at a dilution of 1:2,000 in PBS-Tween 20 for 2 h. After washing for 1 h with PBS, the grids were stained with 10-nm gold-conjugated anti-rabbit antibody (Pelco, Redding, CA). Grids were stained with uranyl acetate and scanned with a JEOL 100CX transmission electron microscope.
Immunohistochemistry.
Mammary glands of wild-type and homozygous mice were embedded in Histo Prep (Fisher Scientific) and frozen at −20°C. Ten-micrometer sections were cut with a Cryostat (Leica) at −20°C and picked up on ProbeOn Plus charged and precleaned microscope slides (Fisher Scientific). Sections were incubated with 1:2,000 dilutions of goat polyclonal antibodies against mouse β-casein (S-15, Santa Cruz Biotechnology) for 2 h at room temperature. After washing, staining was performed with Alexa Fluor 350-tagged anti-rabbit antibody. Sections were examined in Axioplan 2 microscope (Zeiss), and images were captured with an AXIOCam HRc digital camera (Zeiss) under appropriate filters.
AFM.
Milk from the heterozygous and wild-type mice was collected on postpartum day 11, defatted, and diluted 1:200 with water. The diluted milk was placed on freshly cleaved mica and dried before scanning with a Bioscope microscope with a Nanoscope IV controller (Digital Instruments, Santa Barbara, CA) in tapping mode using a RTESP probe at 292 kHz with a tip radius of ≈10 nm (spring constant of 20–100 N/m; frequency of 200–400 kHz).
Supplementary Material
Acknowledgments
We thank Prof. Douglas Dalgleish and Bony de Kumar for discussions; Dr. Andreas Kolb for providing anti-casein antibodies; Jyothi Lakshmi, Manish Mishra, and Purnima Sailasree for excellent technical assistance; and Lalji and Hari for support. This work was supported by the Department of Biotechnology, the Government of India, the Department of Science and Technology, the Government of India, and the Council of Scientific and Industrial Research (New Delhi).
Abbreviations
- dpc
days postcoitum
- ER
endoplasmic reticulum
- AFM
atomic force microscopy.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Davis D. T., Law A. J. R. J. Dairy Res. 1980;47:83–90. [Google Scholar]
- 2.Rijnkels M. J. Mammary Gland Biol. Neoplasia. 2002;7:327–345. doi: 10.1023/a:1022808918013. [DOI] [PubMed] [Google Scholar]
- 3.Bonsing J., Mackinlay A. G. J. Dairy Res. 1987;54:447–461. doi: 10.1017/s0022029900025632. [DOI] [PubMed] [Google Scholar]
- 4.Jolles P., Loucheux-Lefebvre M. H., Henschen A. J. Mol. Evol. 1978;11:271–277. doi: 10.1007/BF01733837. [DOI] [PubMed] [Google Scholar]
- 5.Thompson M. D., Dave J. R., Nakhasi H. L. DNA. 1985;4:263–271. doi: 10.1089/dna.1985.4.263. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki K., Weiss K. M. Proc. Natl. Acad. Sci. USA. 2003;100:4060–4065. doi: 10.1073/pnas.0638023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S., Clarke A. R., Hooper M. L., Horne D. S., Law A. J. R., Leaver J., Springbett A., Stevenson E., Simons P. Proc. Natl. Acad. Sci. USA. 1994;91:6138–6142. doi: 10.1073/pnas.91.13.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanat E., Martin P., Ollivier-Bousquet M. J. Cell Sci. 1999;112:3399–3412. doi: 10.1242/jcs.112.19.3399. [DOI] [PubMed] [Google Scholar]
- 9.Dalgleish D. G., Horne D. S., Law A. J. R. Biochim. Biophys. Acta. 1989;991:383–387. [Google Scholar]
- 10.Donnelly W. J., McNeill I. G. P., Buchheim W., McGann T. C. A. Biochim. Biophys. Acta. 1984;789:136–143. doi: 10.1016/0167-4838(84)90197-3. [DOI] [PubMed] [Google Scholar]
- 11.Mackinlay A. G., Wake R. G. In: Milk Proteins: Chemistry and Molecular Biology. McKenzie H. A., editor. Vol. 2. New York: Academic; 1971. pp. 175–194. [Google Scholar]
- 12.Kumosinski T. F., Farrell H. M., Jr. J. Protein Chem. 1991;10:3–16. doi: 10.1007/BF01024650. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt D. G. In: Developments in Dairy Chemistry 1. Fox P. F., editor. London: Applied Science; 1982. pp. 61–86. [Google Scholar]
- 14.Holt C., Horne D. S. Neth. Milk Dairy J. 1996;50:85–111. [Google Scholar]
- 15.Zucchi I., Bini L., Albani D., Valaperta R., Liberatori S., Raggiaschi R., Montagna C., Susani L., Barbieri O., Pallini V., et al. Proc. Natl. Acad. Sci. USA. 2002;99:8660–8665. doi: 10.1073/pnas.132259399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgoyne R. D., Handel S. E., Sudlow A. W., Turner M. D., Kumar S., Simons J. P., Blatchford D. R., Wilde C. J. In: Intercellular Signaling in the Mammary Gland. Wilde C. J., editor. New York: Plenum; 1995. pp. 253–263. [Google Scholar]
- 17.Farrell H. M., Jr., Cooke P. H., Wickham E. D., Piotrowski E. G., Hoagland P. D. J. Protein Chem. 2003;22:259–273. doi: 10.1023/a:1025020503769. [DOI] [PubMed] [Google Scholar]
- 18.Farrell H. M., Jr., Malin E. L., Brown E. M., Qi P. X. Curr. Opin. Colloid Interface Sci. 2006 doi: 10.1016/j.cocis.2005.11.005. [DOI] [Google Scholar]
- 19.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., Hotamisligil G. S. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 20.Waugh D. F., Noble R. W., Jr. J. Am. Chem. Soc. 1965;87:2246–2257. doi: 10.1021/ja01088a026. [DOI] [PubMed] [Google Scholar]
- 21.Paquin P., Britten M., Laliberte M. F., Boulet M. In: Proteins at Interfaces. Brash J. L., Horbett T. A., editors. Washington, DC: Am. Chem. Soc.; 1987. pp. 677–686. [Google Scholar]
- 22.Walstra P. J. Dairy Res. 1990;73:1965–1979. doi: 10.1017/s0022029900017234. [DOI] [PubMed] [Google Scholar]
- 23.Horne D. S., Parker T. G., Dalgleish D. G. In: Casein Micelles, Polycondensation and Fractals in Food Colloids. Bee R. D., Richmond P., Mingins J., editors. London: R. Soc. Chem.; 1989. pp. 400–405. Special Publication No. 75. [Google Scholar]
- 24.Slattery C. W., Evard R. Biochim. Biophys. Acta. 1973;317:529–538. doi: 10.1016/0005-2795(73)90246-8. [DOI] [PubMed] [Google Scholar]
- 25.Horne D. S. Int. Dairy J. 1998;8:171–177. [Google Scholar]
- 26.Hiripi L., Baranyi M., Szabo L., Toth S., Fontaine M. L., Devinoy E., Bosze Z. J. Dairy Res. 2000;67:541–550. doi: 10.1017/s0022029900004386. [DOI] [PubMed] [Google Scholar]
- 27.Brooker B. E., Holt C. J. Dairy Res. 1979;46:193–195. doi: 10.1017/s0022029900017039. [DOI] [PubMed] [Google Scholar]
- 28.Mercier J. C., Vilotte J. L. J. Dairy Sci. 1993;76:3079–3098. doi: 10.3168/jds.S0022-0302(93)77647-X. [DOI] [PubMed] [Google Scholar]
- 29.Alexander L. J., Stewart A. F., Mackinlay A. G., Kapelinskaya T. V., Tkach T. M., Gorodetsky S. I. Eur. J. Biochem. 1988;178:395–401. doi: 10.1111/j.1432-1033.1988.tb14463.x. [DOI] [PubMed] [Google Scholar]
- 30.Ginger M. R., Grigor M. R. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999;124:133–145. doi: 10.1016/s0305-0491(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 31.Qi P. X., Wickham E. D., Piotrowski E. G., Fagerquist C. K., Farrell H. M., Jr. Protein J. 2005;24:431–444. doi: 10.1007/s10930-005-7639-6. [DOI] [PubMed] [Google Scholar]
- 32.Schaar J., Hansson B., Pettersson H.-E. J. Dairy Res. 1985;52:429–437. [Google Scholar]
- 33.Brophy B., Smolenski G., Wheeler T., Wells D., L’Huillier P., Laible G. Nat. Biotechnol. 2003;21:157–162. doi: 10.1038/nbt783. [DOI] [PubMed] [Google Scholar]
- 34.Jomini L. A., Sarathi D. P., Goel S., Kumar S. Curr. Sci. 2005;88:1167–1168. [Google Scholar]
- 35.Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Proc. Natl. Acad. Sci. USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds E. S. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bera A., Singh S., Nagaraj R., Vaidya T. Mol. Biochem. Parasitol. 2003;127:23–35. doi: 10.1016/s0166-6851(02)00300-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.