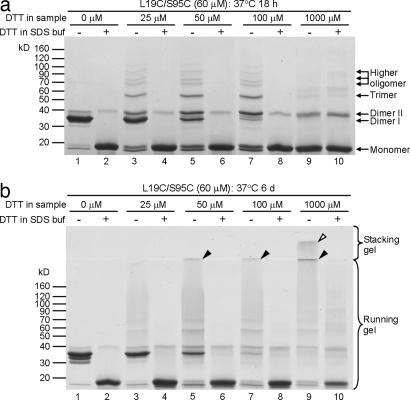
Fig. 6.
Monomers of T7EI L19C/S95C are cross-linked into small oligomers and fibrils by disulfide bonds, demonstrating an organization based on a runaway domain swap. (a) SDS/PAGE of T7EI L19C/S95C incubated at 37°C for 18 h. This gel shows that the fibrils contain higher oligomers (lanes 3, 5, and 7), and these oligomers are disulfide-bridged because they are reduced to monomers by DTT (lanes 4, 6, and 8). The sample with 1,000 μM DTT does not show a lot of higher oligomers (lane 9) because excess DTT is present in the sample. (b) SDS/PAGE of L19C/S95C incubated at 37°C for 6 days. After 6 days at 37°C, the SDS-solubilized fibrils show even higher oligomers that remain at the boundary of the stacking gel and running gel (lanes 5, 7, and 9, filled arrowheads) or on the surface of the stacking gel (lane 9, open arrowhead), and most of these fibrils are converted into monomers by adding DTT in the gel-loading buffer (lanes 6, 8, and 10).