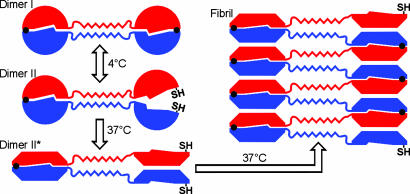
Fig. 7.
Schematic model for the fibril formation of T7EI L19C/S95C. Each subunit is colored either in red or blue. Black dots represent disulfide bonds, and SH represents free cysteine. In dimer I, both disulfide bonds are intact and the protein is locked in close-ended dimers and unable to form fibrils. In dimer II, half of the domain-swapped molecule is unlatched by the reduction of one of the two disulfide bonds. Upon incubation at 37°C, dimer II changes to an open form (denoted as dimer II*), in which half of the dimer opens up, exposing the interface that remains protected in dimer I. Open-ended dimer II* readily fibrillizes via runaway domain swapping. The hinge-loop region of the domain-swapped protein forms a zipper spine in the fibrils.