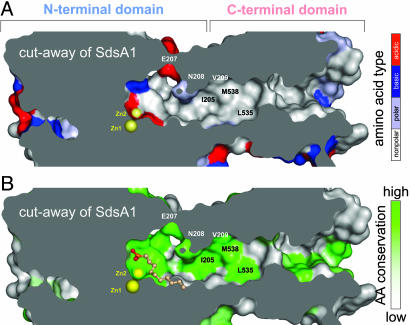
Fig. 4.
The hydrophobic chute connecting the C-terminal domain with the active site of SdsA1. The van der Waals surface of SdsA1 (colored) has been cut away to reveal the hydrophobic chute; the molecular interior is colored gray. Zn ions (yellow spheres) mark the active site. Selected amino acids are marked. (A) Predominantly hydrophobic residues (gray) line the chute, whereas polar (light blue), basic (blue), and acidic (red) amino acids mark the active site or the molecular surface. (B) Amino acids lining the chute are strongly conserved (green) in contrast to the molecular surface (gray). Bound 1DA is shown in ball-and-stick representation.