Abstract
Genomic material from chromosome band 13q14.3 distal to the retinoblastoma locus is recurrently lost in a variety of human neoplasms, indicating an as-yet-unidentified tumor-suppressor mechanism. No pathogenic mutations have been found in the minimally deleted region until now. However, in B cell chronic lymphocytic leukemia tumors with loss of one copy of the critical region, respective candidate tumor-suppressor genes are down-regulated by a factor >2, which would be expected by a normal gene-dosage effect. This finding points to an epigenetic pathomechanism. We find that the two copies of the critical region replicate asynchronously, suggesting differential chromatin packaging of the two copies of 13q14.3. Although we also detect monoallelic silencing of genes localized in the critical region, monoallelic expression originates from either the maternal or paternal copy, excluding an imprinting mechanism. DNA methylation analyses revealed one CpG island of the region to be methylated. DNA demethylation of this CpG island and global histone hyperacetylation induced biallelic expression, whereas replication timing was not affected. We propose that differential replication timing represents an early epigenetic mark that distinguishes the two copies of 13q14.3, resulting in differential chromatin packaging and monoallelic expression. Accordingly, deletion of the single active copy of 13q14.3 results in significant down-regulation of the candidate genes and loss of function, providing a model for the interaction of genetic lesions and epigenetic silencing at 13q14.3 in B cell chronic lymphocytic leukemia.
Keywords: DNA methylation, monoallelic expression, replication timing
B cell chronic lymphocytic leukemia (B-CLL) is the most frequent leukemia of adults in the Western world, and >50% of patients show loss of one copy of a critical region of ≈400 kbp in chromosomal band 13q14.3 (1, 2). This critical region is also recurrently deleted in other hematological malignancies and a variety of solid tumors (reviewed in ref. 3). Accordingly, a tumor-suppressor mechanism has been postulated in this region. Despite extensive efforts, no mutations of potential pathogenic significance have been found in the candidate genes or in intergenic regions of the remaining chromosome copy until now (2, 4, 5). However, the majority of genes localized within and in the vicinity of the critical region, including miR15 and miR16, are down-regulated in CLL with monoallelic deletions by a factor of >2, which would be expected for a simple gene-dosage effect. This substantial down-regulation upon loss of one gene copy points to an epigenetic pathomechanism (3, 6, 7). Intergenic regions of chromosomal band 13q14.3 are conserved in the syntenic region in mice, suggesting conservation of regulatory elements (8). Mosaic deletion patterns were found in B-CLL tumors (2, 9), indicating a multigenic and complex mechanism. Furthermore, the molecular features of the critical region are reminiscent of known imprinted loci that are subject to parent-of-origin-specific monoallelic expression. Two large, noncoding RNA (ncRNA) genes (BCMS and BCMSUN/DLeu2/RFP2OS) span the entire region (10, 11) (Fig. 1A), and a gene localized 2.5 Mbp proximal to the critical region is imprinted in a subpopulation of healthy probands (HTR2A) (12). Given these findings, we investigated whether the candidate genes are monoallelically expressed and whether the critical region is imprinted in lymphatic tissues by testing for parent-of-origin-specific expression. Furthermore, we analyzed epigenetic features of the critical region by measuring DNA replication timing, DNA methylation of CpG islands, and transcriptional activity upon treatment with inhibitors of DNA methyltransferases and histone deacetylases. We propose a model for a tumor-suppressor pathomechanism in which deletion of the single, active chromosome copy results in complete loss of tumor-suppressor function in the critical region.
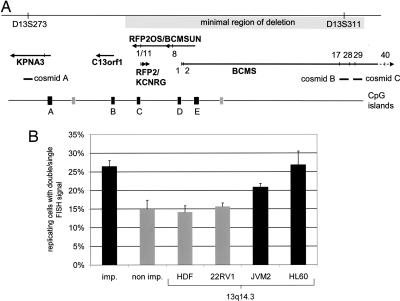
Fig. 1.
The critical region in chromosomal band 13q14.3 replicates asynchronously in cell lines. (A) Overview of the critical region between the genomic markers D13S273 and D13S311. The minimal region of deletion is shown as a long gray box. Genes and their direction of transcription are depicted as arrows. Perpendicular lines mark exons (BCMS and BCMSUN/DLEU2/RFP2OS) or SNPs (other genes) that were analyzed. Cosmids are delineated as horizontal bars. CpG islands identified in the region applying stringent conditions (24) are shown as black boxes when they are associated with 5′ ends of genes and as gray boxes when they are not. (B) Three cosmids localized in 13q14.3 (A, B, and C in A) were hybridized to interphase nuclei and compared with genomic probes localizing to an imprinted locus (imp.) and a nonimprinted locus (non imp.). Genomic probes were hybridized to nuclei of hematopoietic cell lines (HL60 and JVM-2) and nonhematopoietic cells (HDF and 22RV1). For each probe and cell line, 200 BrdU-positive cells were screened for asynchronously replicating cells with one single and one double signal (n = 4,000 cells) (for representative fluorescence in situ hybridization images, see Fig. 7). The proportion of cells showing asynchronous replication timing is depicted. Error bars give averages of the four cell lines tested (control loci) or of cosmids A, B, and C, which localize in 13q14.3.
Results
The Critical Region in 13q14.3 Replicates Asynchronously in Hematopoietic Cell Lines.
In imprinting, discrimination of the two gene copies is achieved through epigenetic chromatin modifications, such as differential DNA methylation (13), histone modifications (14), and replication timing (15). We visualized asynchronous replication timing by fluorescence in situ hybridization under experimental conditions that separate replicated chromatids (16, 17). Imprinted regions have been shown to replicate asynchronously (17, 18), with the fraction of asynchronously replicating cells ranging from 21% to 41%, depending on the region analyzed and cell line used. In contrast, nonimprinted regions have shown asynchronous replication in only 9–19% of cells. In line with these results, we used a region on chromosome 20 well known for being imprinted as positive control (NNAT) (19) and found asynchronous replication in >20% of cells, as expected (28.6% in HDF, 27.3% in 22RV1, 24.5% in JVM-2, and 25.5% in HL60 cells; see “imp.” in Fig. 1B). A locus on chromosome 22 that contains no imprinted gene (19) was used as negative control and showed asynchronicity only in 15.0 ± 2.3% of cells (15.5% in HDF, 11.5% in 22RV1, 15.0% in HL60, and 18.0% in JVM-2 cells; see “non imp.” in Fig. 1B). For 13q14.3 cosmid clones A, B, and C (for localization, see Fig. 1A), we found >20% of cells replicated asynchronously in hematopoietic cell lines (19.5%, 21.0%, and 22.0% in JVM2 and 22.5%, 26.5%, and 31.5% in HL60), a value corresponding to the imprinted control locus (Fig. 1B). In contrast, nonhematopoietic cell lines HDF and 22RV1 replicated 13q14.3 asynchronously only in 14.2 ± 1.6% and 15.7 ± 0.8% of cells, respectively. Thus, asynchronous replication timing of 13q14.3 occurs at a similar frequency as in an imprinted region and more frequently than at a nonimprinted control region (P < 0.01, Mann–Whitney U test) and than in nonhematopoietic cell lines (P < 0.005). An explanation for the fact that the critical region at 13q14.3 does not replicate asynchronously in nonhematopoietic cell lines could be either the loss of asynchronous replication timing in the cell lines used or restriction of asynchronous replication timing to hematopoietic cells only.
Candidate Tumor-Suppressor Genes Are Monoallelically Expressed in Peripheral Blood Lymphocytes of Healthy Probands and Hematopoietic Cell Lines.
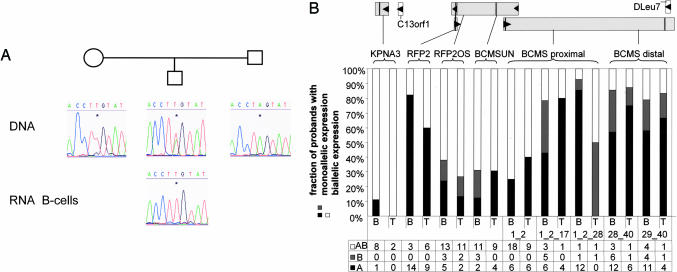
Asynchronous replication timing at 13q14.3 may be due to differential chromatin packaging of the two copies of the critical region and may thereby influence transcriptional activity of the genes in the critical region. Genome-wide screens discovered monoallelic expression in human cells in a surprisingly large number of genes by using analysis of single-nucleotide polymorphisms (SNPs) in RNA (20, 21). To investigate whether both gene copies are actively transcribed, we screened 13q14.3 genes for SNPs localized in candidate genes of the critical region that would allow discrimination of the two alleles (Table 1, which is published as supporting information on the PNAS web site). BCMSUN/DLeu2 shows very high homology to BCMSUNL localized in chromosomal band 1p22 (22), and the BCMSUN/Dleu2 SNP used for analysis is localized in the terminal 75 base pairs specific for 13q14.3. Exon 11 of RFP2OS/BCMSUN overlaps with RFP2 in the opposite direction over 144 base pairs localized in the first exon of RFP2, and the SNP in exon 1L of RFP2 is localized within these 144 base pairs. After identifying healthy probands heterozygous for one or more of these SNPs, B and T cells were isolated from these probands, and the candidate tumor-suppressor genes of 13q14.3 were tested for monoallelic expression by RT-PCR amplification of the SNPs and subsequent sequence analysis (Fig. 2A). Intriguingly, we detected monoallelic expression of five of six genes localized in 13q14.3. Whereas KPNA3 was biallelically expressed in eight of nine probands, RFP2 and splicing variants of BCMS containing distal exons were monoallelically expressed in >80% of probands analyzed (Fig. 2B). In 25–40% of healthy probands, RFP2OS, BCMSUN, and the most proximal splicing variant of BCMS were also monoallelically expressed. Monoallelic expression was present both in B and T cells of healthy probands, even though fewer T cell samples were available for testing. Because of the paucity of B-CLL-derived cell lines, we genotyped 3 B-CLL cell lines and 14 additional cell lines for heterozygosity at the 13q14.3 SNPs and tested heterozygous cell lines for monoallelic expression (Table 2, which is published as supporting information on the PNAS web site). Similar to probands, RFP2 and BCMS were monoallelically expressed in one of two and six of six informative cell lines tested, respectively, whereas RFP2OS and BCMSUN were biallelically expressed in two of two and four of four cell lines tested, respectively. Interestingly, even cell lines with four copies of the critical region (Jurkat and MOLT-4) expressed only one copy of RFP2 and BCMS, respectively (Table 2). The latter finding means that monoallelic expression at 13q14.3 persisted through polyploidization, pointing to a robust regulatory mechanism that is retained independently of chromosome copy number. In summary, we show monoallelic expression of 13q14.3 genes that are candidate tumor-suppressor genes in B and T cells of healthy probands and hematopoietic cell lines.
Fig. 2.
Candidate tumor-suppressor genes in the critical region of chromosomal band 13q14.3 are monoallelically expressed in healthy probands. (A) Cells from probands heterozygous for at least one SNP were tested for monoallelic expression of candidate genes. In addition, parents of heterozygous probands were genotyped to assess the origin of the expressed allele. (B) Five genes (KPNA3, RFP2, RFP2OS, BCMSUN, and BCMS) were tested for monoallelic expression in sorted B or T cells of healthy probands. (Upper) Genes analyzed are depicted as gray boxes, arrows show the direction of transcription, and vertical lines within the genes denote the position of SNPs. For RFP2 and RFP2OS, the same SNP in exon 1L of RFP2 and exon 1 of RFP2OS was used. For BCMS, two SNPs in exons 1 and 40 were used for all splicing variants containing exons 1 and 2; 1, 2, and 17; 1, 2, and 28; 28 and 40; or 29 and 40, which are the most commonly transcribed variants of BCMS (10). (Lower) Sorted B and T cells (B and T, respectively) from probands heterozygous for at least one SNP were analyzed. Shown are percentages and absolute numbers of probands expressing both alleles (AB), the more frequently expressed allele (A), or the less frequently expressed allele (B).
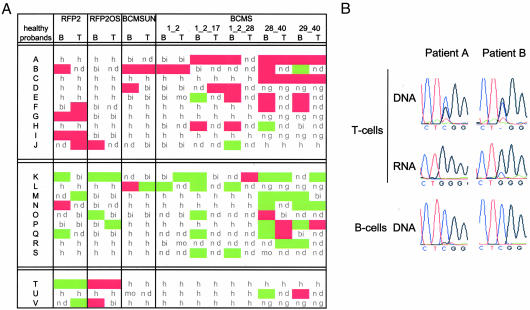
Monoallelic Expression of the Paternal or the Maternal Chromosome Copy in Healthy Probands Excludes Imprinting of the Critical Region.
The molecular make-up of 13q14.3 with the presence of long ncRNA genes spanning the entire critical region and imprinting of the HTR2A gene localized in the vicinity is reminiscent of imprinted regions. Therefore, we tested whether the transcriptionally active chromosome is always of the same parental origin by genotyping parents for the respective SNPs. In the case of imprinting, expression would always derive from the same parental gene copy, i.e., always from either the paternal or maternal copy in all probands. In contrast, we found for every monoallelically expressed gene a similar number of probands expressing either the paternal or maternal gene copy of 13q14.3 (Fig. 3A). This finding excludes imprinting at the critical region in B and T cells. However, when looking at single probands, a highly imbalanced chromosome usage was observed either with the maternal chromosome preferentially expressed or the paternal chromosome (Fig. 3A). Mainly the maternal chromosome was active in probands A–J (n = 10), whereas it was mainly the paternal chromosome that was active in K–S (n = 9). No clear preference could be detected in probands T–V (n = 3). These findings indicate that a subgroup of genes of the critical region in 13q14.3 are expressed from one chromosome copy only and are silenced on the other copy. This variance in silencing has also been detected in other genomic regions (20, 21). Because monoallelic expression of candidate genes occurred preferentially only from one chromosome copy, but not exclusively, we tested, by a statistical approach, whether such a preferential chromosome usage could occur by chance. We computed 1,000 random patterns, each consisting of 22 probands and 16 genes, splicing variants, and tissues We took into account the distribution of heterozygosity and monoallelic expression that we had detected in our set of 126 probands (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). Only 8 of 1,000 random patterns showed a similar preferential expression of the same chromosome. It is therefore highly unlikely (P = 0.008) that the preferential usage of the same copy of 13q14.3, which we detected in healthy probands, occurred by chance.
Fig. 3.
Monoallelically expressed candidate genes are not imprinted but expressed from the same chromosome copy, and this active copy is deleted in CLL. (A) Parents of probands heterozygous for at least two of the tested SNPs were genotyped, and the monoallelically expressed allele of the offspring was scored for parental origin (maternal expression, green; paternal expression, red). Also shown are biallelically expressed genes (bi), monoallelically expressed genes for which parents were not available or not informative (mo), loci where the proband was homozygous (h), loci where gene expression was below the detection limit (nd), and loci where the proband was not genotyped (ng). (B) Peripheral blood lymphocytes of two CLL patients with monoallelic deletion of 13q14.3, which were informative for a SNP in RFP2OS, were sorted into a CD19-positive fraction containing mostly malignant cells (B cells) and a CD19-negative fraction containing mostly nonmalignant cells (T cells). The copy of RFP2OS active in the nonmalignant fraction is deleted in the malignant cells.
The Active Copy of RFP2OS Is Deleted in CLL Patients.
Monoallelic expression of the critical region has profound consequence for the pathomechanism of 13q14.3, because loss of the active gene copy would already suffice to abolish gene function. To test this model, we isolated T cells from CLL probands heterozygous for the SNP localized in RFP2OS (Fig. 3B Top). In these nonmalignant T cells, we identified the active copy of RFP2OS (Fig. 3B Middle) and then tested the B cells that represent the CLL tumor for loss of the active gene copy. Consistent with our model, in two of two informative cases, the gene copy of RFP2OS that was active in T cells was deleted in B cells (Fig. 3B Bottom).
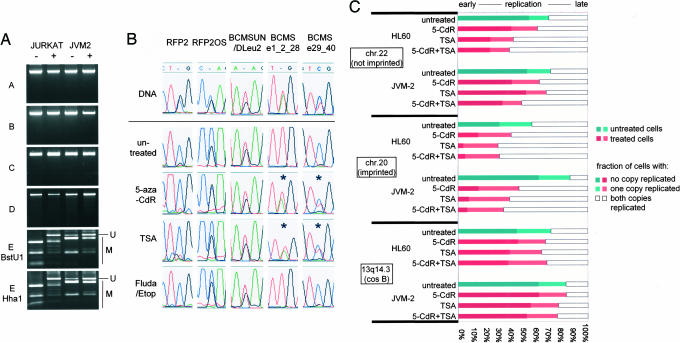
Only One CpG Island of 13q14.3 Is DNA-Methylated in Cell Lines.
Because the critical region in 13q14.3 shows gene down-regulation in tumors (6, 7), asynchronous replication timing, and monoallelic expression, the presence of an epigenetic modification, such as DNA methylation, was very likely. In allelic exclusion of the immunoglobulin κ genes, for example, one allele is silenced and not recombined during the generation of antigen receptors. This silent gene copy is characterized by methylated DNA and deacetylated histones (23). The use of stringent search criteria (24) resulted in the identification of seven CpG islands in the critical region (Fig. 1A). We have analyzed four of these CpG islands previously by using a methylation-specific PCR (MSP)-based method (6). However, MSP is restricted to the analysis of CpGs spanned by the PCR primers and allows only relative quantitation. Therefore, we performed a more detailed analysis of DNA methylation of five CpG islands that colocalize with 5′ ends of candidate genes by using combined bisulfite and restriction analysis (25) and bisulfite sequencing (26). Of the five CpG islands analyzed, only CpG island E (see Fig. 1A) was methylated, with combined bisulfite and restriction analysis showing almost complete methylation in Jurkat and only intermediate methylation in JVM-2 cells (Fig. 4A). The same results were obtained with bisulfite sequencing (Fig. 5, which is published as supporting information on the PNAS web site, and data not shown). Interestingly, CpG island E is localized right next to the two large ncRNA genes (BCMSUN/RFP2OS and BCMS) that have been postulated to regulate other genes of the critical region in 13q14.3 (3).
Fig. 4.
Only one CpG island in the critical region is DNA-methylated, and euchromatinization results in reexpression of the silent gene copy but no change in replication timing. (A) By using combined bisulfite and restriction analysis, DNA methylation was analyzed in CpG islands localized in 13q14.3. DNA methylation was detected in only one of five CpG islands in the critical region (see CpG island E in Fig. 1A). Upon treatment with 5-aza-CdR, DNA methylation was reduced. −, untreated cells; +, cells treated with 5-aza-CdR; U, bands representing unmethylated DNA; M, bands representing methylated DNA. (B) Different cell lines heterozygous for at least one SNP were incubated with 5-aza-CdR or TSA for 24 h and analyzed for monoallelic or biallelic expression. To rule out effects due to apoptosis, cells were also incubated with either fludarabine or etoposide. Whereas RFP2, RFP2OS, and BCMSUN did not change expression upon euchromatization in one of one and three of three cell lines tested, monoallelic silencing of the distal exons of BCMS was reduced and expression occurred from both gene copies in two of three cell lines tested. Shown are representative results of RFP2 and RFP2OS in Jurkat, BCMSUN in JVM-2, and BCMS in JVM-2 and Jurkat. (C) JVM2 and HL60 cells were treated with 5-aza-CdR or TSA, and replicating cells were scored for two unreplicated chromatids (SS), one replicated and one unreplicated chromatid (SD), or a completely replicated locus (DD). Two control loci from a nonimprinted (chromosome 22q12, 35.5 Mbp) and an imprinted region (NNAT, chromosome 20q11.2, 35.3 Mbp) shift to earlier replication after treatment with 5-aza-CdR and TSA due to euchromatization. In contrast, replication timing of 13q14.3 did not change after treatment with 5-aza-CdR or TSA.
Inhibitors of DNA Methyltransferases and Histone Deacetylases Reduce Silencing at 13q14.3 in Cell Lines but Do Not Affect Replication Timing.
To elucidate the role of DNA methylation of CpG island E associated with the ncRNA genes in 13q14.3, we wanted to test whether methylation of DNA or acetylation of histones are involved in the silencing effects at 13q14.3. It has been shown that treatment with inhibitors of DNA methyltransferases [e.g., 5-aza-2′-deoxycytidine (5-aza-CdR)] or histone deacetylases [e.g., trichostatin A (TSA)] leads to euchromatinization in cell culture (27). We assayed 5-aza-CdR for its efficacy in global DNA demethylation in cell lines and could reduce whole-genome methylation of cytosine significantly compared with untreated cells (Fig. 6, which is published as supporting information on the PNAS web site). Consistently, treatment with 5-aza-CdR also caused detectable demethylation of CpG island E in Jurkat and JVM-2 cells (Fig. 4A). Bisulfite sequencing of demethylated Jurkat cells detected two significantly distinct types of DNA methylation of this CpG island (P < 0.003, Mann–Whitney U test; see Fig. 5). This pattern suggests allelic demethylation, which points to different chromatin packaging of the two copies of CpG island E. Subsequent analysis of gene expression showed that treatment with 5-aza-CdR did not alleviate monoallelic silencing of RFP2 and did not alter biallelic expression of testable splice variants of RFP2OS or BCMSUN in one of one or three of three cell lines tested, respectively (Jurkat and JVM-2, HL60, and Jurkat) (Fig. 4B). One explanation for this phenomenon is an absence of DNA methylation of the CpG islands A–D associated with the 5′ ends of KPNA3, C13ORF1, RFP2, and BCMS in untreated cells. Another possibility is that RFP2 is silenced by an epigenetic mark not affected by 5-aza-CdR or TSA in cell culture (i.e., replication timing; see below). In contrast, demethylation of CpG island E correlated with biallelic expression of BCMS splicing variants that include distal exons in two of three cell lines analyzed (JVM2 and Jurkat, but not HL60) (Fig. 4B). A similar effect could also be observed after treatment with the histone deacetylase inhibitor TSA (Fig. 4B), which causes histone hyperacetylation and reactivation of heterochromatin. Thus, silencing of one copy of BCMS involves DNA methylation and histone deacetylation. Next, we wanted to determine whether 5-aza-CdR and TSA would change replication timing of the critical region in 13q14.3. Assays for replication timing on a genome-wide scale have suggested a model of preferential recruitment of the replication machinery to open chromatin (28). Thus, we expected that opening of the chromatin by global DNA demethylation and histone hyperacetylation would shift replication timing to earlier S phase. As predicted, hybridization with a control locus on chromosome 22 resulted in fewer nonreplicated cells, with two single signals after treatment with 5-aza-CdR, TSA, or both. This result means that the cells were shifted toward earlier replication timing at this locus after treatment (Fig. 4C). This shift was even more pronounced in the imprinted region on chromosome 20 (Fig. 4C). To our surprise, replication timing at 13q14.3 did not change after demethylation of DNA, histone hyperacetylation, or both (Fig. 4C).
Discussion
Epigenetic and Genetic Effects Interact at the Tumor-Suppressor Locus 13q14.3.
Currently, there are three mechanisms of monoallelic expression described in humans: (i) preferential expression of specific SNP alleles, (ii) imprinting, and (iii) allelic exclusion. Except for RFP2, we found no preferential expression of a specific SNP allele (data not shown). We exclude imprinting at 13q14.3, because imprinted genes are only expressed from the same parental chromosome in all individuals, whereas we found candidate genes to be silenced either on the maternal or paternal chromosome copy of 13q14.3. We also show that the region replicates asynchronously, and this asynchronous replication timing is indifferent to chromatin remodeling agents. These results are reminiscent of allelic exclusion of Ig receptor genes. In allelic exclusion, asynchronous replication timing is the first marker distinguishing the two copies of the Ig genes and results in their differential nuclear localization, DNA methylation, histone modification, transcriptional silencing, and, finally, nonrecombination (reviewed in ref. 29). At the Ig loci, asynchronous replication timing is independent of the DNA methylation status (17) and transcriptional activity (16). Even engineered rearrangement of Ig loci, which induces changes in chromatin packaging, does not change their asynchronous replication timing (16). Our findings in the critical region of 13q14.3 are similar to the Ig loci in that expression can at least partially be modulated by demethylation of CpG island E. Also, replication timing of 13q14.3 is neither changed by global demethylation of DNA nor by histone hyperacetylation, placing it upstream of DNA methylation of CpG island E and gene expression. A major difference between 13q14.3 and allelic exclusion is usage of the same copy of 13q14.3 in the whole tissue, whereas, in allelic exclusion, the silencing decision is taken in each cell independently, resulting in quantitatively similar expression of both copies when the entire tissue is analyzed. At 13q14.3, silencing is independent of the parental origin, but the same chromosome copy is inactivated in the whole tissue. We find either the maternal or paternal copy of 13q14.3 active in different healthy probands, meaning that every cell in the whole tissue has the same copy silenced. In addition, we detected a strong bias toward activity of the same chromosome copy both in B and T cells (Fig. 3A). This finding suggests that silencing takes place at a very early developmental stage and can be stably maintained through many cell divisions so that, both in B and T cells, the same gene copy is silenced. Replication timing of the critical region remained stable, even if expression was forced from the silenced chromosome by global demethylation of DNA and histone hyperacetylation. Thus, we suggest that, similar to allelic exclusion, asynchronous replication timing represents an early epigenetic mark discriminating both chromosomes at 13q14.3. Asynchronous replication timing results in differential chromatin packaging of the two copies and methylation of CpG island E and leads to monoallelic expression of specific genes of 13q14.3. The direct consequence of DNA methylation of CpG island E is monoallelic expression of the distal variants of the BCMS ncRNA gene, because demethylation of this CpG island leads to reexpression of the silenced gene copy (Fig. 4B). However, we cannot exclude the possibility that specific splicing variants of the second ncRNA gene BCMSUN/DLEU2 are also monoallelically silenced by methylation of CpG island E, because the only testable splicing variant for BCMSUN/DLEU2 was biallelically expressed in all informative cell lines tested (Table 2). The intriguing question remains: Why is only one copy of the critical region active in healthy probands? In allelic exclusion, one allele is silenced to ensure that the complex process of Ig gene recombination takes place only once. In 13q14.3, others and we observed excessive splicing of all large ncRNA genes (3, 10, 11). Because only genes from one of the chromosomes are expressed in 13q14.3, it is tempting to speculate that transcriptional and posttranscriptional complexity of the critical region requires silencing of the second chromosome similar to the Ig locus.
A Model for the Role of Monoallelic Expression at 13q14.3 in B-CLL.
We show here that genes in the critical region of 13q14.3 are monoallelically expressed in normal B and T cells and that the other gene copy is epigenetically silenced. Hence, loss of the active copy would suffice to completely abolish gene function. As a matter of fact, monoallelic loss of 13q14.3 is the most common aberration in B-CLL patients, but no point mutations have been found in the second chromosome copy. Monoallelic silencing in healthy probands would provide a plausible explanation for the pathogenicity of monoallelic loss of the critical region. Consistent with this model, loss of one gene copy results in almost complete down-regulation of candidate and microRNA genes in B-CLL tumors (3, 6, 7), rather than down-regulation by a factor of 2, as would be expected for a simple gene-dosage effect. In addition, we show deletion of the active copy of RFP2OS in two of two CLL tumors (Fig. 3B). However, biallelic loss of the critical region has also been detected in a subpopulation of B-CLL tumors (2), which is consistent with a subset of healthy probands showing biallelic expression at 13q14.3 (Fig. 2B). In individuals with a biallelic expression, de novo inactivation of both alleles would be required for complete inactivation, resulting in the occurrence of biallelic deletions in a subset of B-CLL tumors. Alternatively, low-level expression or reexpression of the silenced copy upon loss of the active gene copy in B-CLL cells could require deletion of the second allele. This finding is in line with results of long-term follow-up studies of B-CLL tumors with a monoallelic deletion of 13q14.3, where subsequent loss of the second copy of the critical region can be detected after several years (S.S., unpublished work). Also, in a number of B-CLL tumors with a 13q14.3 deletion, not all tumor cells show loss of genomic material from the critical region. This finding supports a model of consecutive inactivation of both gene copies of 13q14.3 by a series of different mechanisms, first of epigenetic nature and then by genetic mechanisms, resulting in a final complete inactivation of the tumor-suppressor mechanism in the region.
In conclusion, we provide experimental evidence for a model that explains why no point mutations can be found in the second allele in B-CLL tumors and how, in the majority of B-CLL tumors, monoallelic deletion is sufficient for complete loss of tumor-suppressor function. Because monoallelic loss of 13q14.3 is the most common genetic aberration detected in B-CLL, inactivation of the active chromosome copy of the critical region by epigenetic mechanisms or deletion would be the initiating event in the tumorigenesis of this leukemia.
Materials and Methods
Cells.
Healthy probands were selected after informed consent (n = 126; median age, 34 yr; range, 23–68 yr; male/female ratio, 1.19) and genotyped by using, as PCR template, either spittle samples or genomic DNA derived from peripheral blood lymphocytes. Peripheral blood lymphocytes were isolated and separated into CD19+ (B cell−) and CD19− (T cell−) fractions as described in ref. 6. Purity of cell fractions was measured by FACS analysis and ranged from 69 to 99% (median, 90.5%). Accession numbers of cell lines used are listed in Supporting Materials and Methods.
Replication Timing.
Replication timing was measured by using harsh conditions to separate all replicated chromatids, as described in refs. 16 and 17, which allowed direct assessment of replication timing of a specific region of interest (Fig. 7, which is published as supporting information on the PNAS web site). Probes were RPCIP704E151141Q (imprinted) and LL22NC01–132D12 (nonimprinted), and cosmids A, B, and C were ICRFc108J1711Q, −B237QD2, and −D0653QD2, respectively. Jurkat and MOLT-4 have four copies of the critical region, excluding them for analysis of replication timing.
RNA Isolation, PCR, and Sequencing.
RNA was isolated by using TRIzol (Invitrogen), reverse transcribed by using SuperScript II (Invitrogen), and amplified with Advantage cDNA polymerase mix (BD Clontech) (for primers, see Table 3, which is published as supporting information on the PNAS web site, and ref. 6). Cleaned PCR products (BioCat, Heidelberg) were sequenced with BIGDYE 3.1 kit on a 3100 Genetic Analyzer (both from Applied Biosystems) and analyzed with the New Staden Package (http://staden.sourceforge.net). To exclude bias in PCR, reactions were repeated at least three times for selected samples and five times for samples in which sufficient material was available. Monoallelic expression was only scored when no expression of the second allele could be detected. To verify quantitative reproduction of allelic expression by PCR and sequencing, we mixed different ratios of DNA from cell lines homozygous for 13q14.3 SNPs and performed PCR and sequencing (Fig. 8, which is published as supporting information on the PNAS web site). Different DNA ratios were reliably represented by sequencing.
Bisulfite Treatment and Combined Bisulfite Restriction Analysis.
DNA was prepared with the DNeasy tissue kit (Qiagen, Hilden, Germany) and subjected to bisulfite treatment as described in ref. 26. Bisulfite-treated DNA was PCR-amplified and either digested with BstUI (New England Biolabs) or cloned, and single clones were sequenced.
DNA Demethylation and Histone Hyperacetylation.
Cells were incubated in medium supplemented with 30, 100, or 150 ng/ml TSA (Sigma) for 24 h or 500 nM to 10 μM 5′-aza-CdR for 3 days (Sigma). Whole-genome 5-methyl-cytosine levels were determined as described in ref. 30 (Fig. 6).
Detailed materials and methods are published in Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank A. Benner and M. Rehmsmeier for statistical and computational analyses, E. Brückle and A. Habermann for excellent technical support and materials, and B. Radlwimmer for helpful discussions. We also thank all of the volunteers who donated samples. This work was supported by Deutsche Forschungsgemeinschaft Grant STI 296/1-1, Sander Stiftung Grant 2002.095.1, and José Carreras International Leukemia Foundation Grant R 04/02.
Abbreviations
- 5-aza-CdR
5′-aza-2′-deoxycytidine
- B-CLL
B cell chronic lymphocytic leukemia
- ncRNA
noncoding RNA
- SNP
single-nucleotide polymorphism
- TSA
trichostatin A.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The SNP data reported in this paper (SNPs in RFP2 and RFP2OS) have been deposited in the National Center for Biotechnology Information (NCBI) Single Nucleotide Polymorphism Database (dbSNP) Build 126 (dbSNP ID no. rs49785043).
References
- 1.Liu Y., Szekely L., Grander D., Soderhall S., Juliusson G., Gahrton G., Linder S., Einhorn S. Proc. Natl. Acad. Sci. USA. 1993;90:8697–8701. doi: 10.1073/pnas.90.18.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stilgenbauer S., Nickolenko J., Wilhelm J., Wolf S., Weitz S., Döhner K., Boehm T., Döhner H., Lichter P. Oncogene. 1998;16:1891–1897. doi: 10.1038/sj.onc.1201764. [DOI] [PubMed] [Google Scholar]
- 3.Corcoran M. M., Hammarsund M., Zhu C., Lerner M., Kapanadze B., Wilson B., Larsson C., Forsberg L., Ibbotson R. E., Einhorn S., et al. Genes Chromosomes Cancer. 2004;40:285–297. doi: 10.1002/gcc.20046. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Corcoran M., Rasool O., Ivanova G., Ibbotson R., Grander D., Iyengar A., Baranova A., Kashuba V., Merup M., et al. Oncogene. 1997;15:2463–2473. doi: 10.1038/sj.onc.1201643. [DOI] [PubMed] [Google Scholar]
- 5.Migliazza A., Bosch F., Komatsu H., Cayanis E., Martinotti S., Toniato E., Guccione E., Qu X., Chien M., Murty V. V., et al. Blood. 2001;97:2098–2104. doi: 10.1182/blood.v97.7.2098. [DOI] [PubMed] [Google Scholar]
- 6.Mertens D., Wolf S., Schroeter P., Schaffner C., Döhner H., Stilgenbauer S., Lichter P. Blood. 2002;99:4116–4121. doi: 10.1182/blood.v99.11.4116. [DOI] [PubMed] [Google Scholar]
- 7.Calin G. A., Dumitru C. D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapanadze B., Makeeva N., Corcoran M., Jareborg N., Hammarsund M., Baranova A., Zabarovsky E., Vorontsova O., Merup M., Gahrton G., et al. Genomics. 2000;70:327–334. doi: 10.1006/geno.2000.6386. [DOI] [PubMed] [Google Scholar]
- 9.Kalachikov S., Migliazza A., Cayanis E., Fracchiolla N. S., Bonaldo M. F., Lawton L., Jelenc P., Ye X., Qu X., Chien M., et al. Genomics. 1997;42:369–377. doi: 10.1006/geno.1997.4747. [DOI] [PubMed] [Google Scholar]
- 10.Wolf S., Mertens D., Schaffner C., Korz C., Döhner H., Stilgenbauer S., Lichter P. Hum. Mol. Genet. 2001;10:1275–1285. doi: 10.1093/hmg/10.12.1275. [DOI] [PubMed] [Google Scholar]
- 11.Baranova A., Hammarsund M., Ivanov D., Skoblov M., Sangfelt O., Corcoran M., Borodina T., Makeeva N., Pestova A., Tyazhelova T., et al. Gene. 2003;321:103–112. doi: 10.1016/j.gene.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Bunzel R., Blumcke I., Cichon S., Normann S., Schramm J., Propping P., Nothen M. M. Brain Res. Mol. Brain Res. 1998;59:90–92. doi: 10.1016/s0169-328x(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 13.Goldmit M., Bergman Y. Immunol. Rev. 2004;200:197–214. doi: 10.1111/j.0105-2896.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 14.Vu T. H., Li T., Hoffman A. R. Hum. Mol. Genet. 2004;13:2233–2245. doi: 10.1093/hmg/ddh244. [DOI] [PubMed] [Google Scholar]
- 15.Kitsberg D., Selig S., Brandeis M., Simon I., Keshet I., Driscoll D. J., Nicholls R. D., Cedar H. Nature. 1993;364:459–463. doi: 10.1038/364459a0. [DOI] [PubMed] [Google Scholar]
- 16.Mostoslavsky R., Singh N., Tenzen T., Goldmit M., Gabay C., Elizur S., Qi P., Reubinoff B., Chess A., Cedar H., Bergman Y. Nature. 2001;414:221–225. doi: 10.1038/35102606. [DOI] [PubMed] [Google Scholar]
- 17.Gribnau J., Hochedlinger K., Hata K., Li E., Jaenisch R. Genes Dev. 2003;17:759–773. doi: 10.1101/gad.1059603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogan P. K., Seip J. R., White L. M., Wenger S. L., Steele M. W., Sperling M. A., Menon R., Knoll J. H. Hum. Genet. 1998;103:694–701. doi: 10.1007/s004390050893. [DOI] [PubMed] [Google Scholar]
- 19.Morison I. M., Reeve A. E. Hum. Mol. Genet. 1998;7:1599–1609. doi: 10.1093/hmg/7.10.1599. [DOI] [PubMed] [Google Scholar]
- 20.Pastinen T., Sladek R., Gurd S., Sammak A., Ge B., Lepage P., Lavergne K., Villeneuve A., Gaudin T., Brandstrom H., et al. Physiol. Genomics. 2004;16:184–193. doi: 10.1152/physiolgenomics.00163.2003. [DOI] [PubMed] [Google Scholar]
- 21.Lo H. S., Wang Z., Hu Y., Yang H. H., Gere S., Buetow K. H., Lee M. P. Genome Res. 2003;13:1855–1862. doi: 10.1101/gr.1006603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mertens D., Wolf S., Bullinger L., Ohl S., Schaffner C., Döhner H., Stilgenbauer S., Lichter P. Int. J. Cancer. 2000;88:692–697. doi: 10.1002/1097-0215(20001201)88:5<692::aid-ijc2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Mostoslavsky R., Singh N., Kirillov A., Pelanda R., Cedar H., Chess A., Bergman Y. Genes Dev. 1998;12:1801–1811. doi: 10.1101/gad.12.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takai D., Jones P. A. Proc. Natl. Acad. Sci. USA. 2002;99:3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Z., Laird P. W. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frommer M., McDonald L. E., Millar D. S., Collis C. M., Watt F., Grigg G. W., Molloy P. L., Paul C. L. Proc. Natl. Acad. Sci. USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth K. F., Knoch T. A., Wachsmuth M., Frank-Stohr M., Stohr M., Bacher C. P., Muller G., Rippe K. J. Cell Sci. 2004;117:4277–4287. doi: 10.1242/jcs.01293. [DOI] [PubMed] [Google Scholar]
- 28.Woodfine K., Fiegler H., Beare D. M., Collins J. E., McCann O. T., Young B. D., Debernardi S., Mott R., Dunham I., Carter N. P. Hum. Mol. Genet. 2004;13:575. doi: 10.1093/hmg/ddh016. [DOI] [PubMed] [Google Scholar]
- 29.Bergman Y., Cedar H. Nat. Rev. Immunol. 2004;4:753–761. doi: 10.1038/nri1458. [DOI] [PubMed] [Google Scholar]
- 30.Stach D., Schmitz O. J., Stilgenbauer S., Benner A., Döhner H., Wiessler M., Lyko F. Nucleic Acids Res. 2003;31:E2. doi: 10.1093/nar/gng002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.