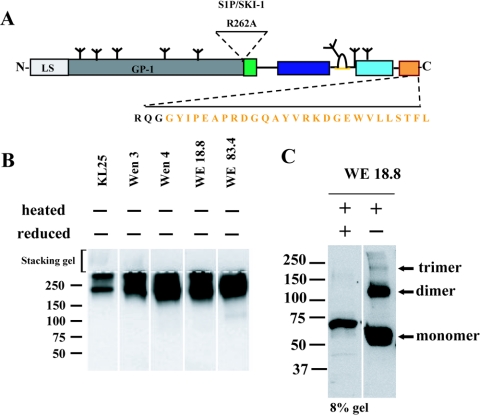
FIG. 4.
T293 cells secrete two distinct GPfib complexes, which contain interchain disulfide bridges. (A) Uncleavable GP fibritin fusion construct. The transmembrane helix and the cytoplasmic tail were replaced by the 27-aa-long trimeric motif of bacteriophage T4-derived fibritin. At the fusion site, two additional glycine residues were introduced to increase the overall flexibility in the linker region. To avoid proteolytic processing, the subtilase SKI-1/S1P consensus sequence was mutated (R262A). HEK T293 cells were stably transfected and subcloned. (B) Nondenaturing Western blot analysis of serum-free cell supernatant. Detection was carried out with the monoclonal antibodies KL25, Wen3, Wen4, WE 18.8, and WE 83.4. KL25, Wen3, and Wen4 bind to the conformational and reduction sensitive epitope GP-1A on GP-1. The antibodies WE 18.8 and WE 83.4 bind to the linear epitopes GP-1C on GP-1 and GP-2A on GP-2, respectively (42). The border of the stacking gel is indicated by a dotted line. Minuses above the blots indicate absence of heat and reduction. (C) Western blot analysis of serum-free cell supernatant with the antibody WE 18.8, when the sample was either completely reduced (lane 1) or heat denatured without reduction (lane 2). Molecular mass markers (in kDa) are noted at the left of blots.