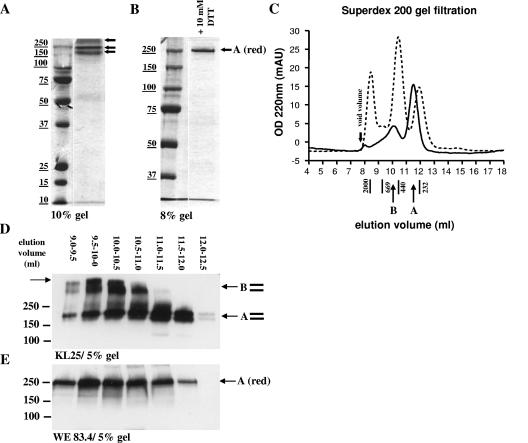
FIG. 5.
GPfib forms two complexes of ∼230 and ∼440 kDa, which upon partial reduction are converted to a single complex species migrating at exactly 250 kDa. GPfib complexes were purified from the cell supernatant by affinity chromatography and analyzed by SDS-PAGE and Coomassie staining. (A) Native nondenatured complexes, when the sample was neither reduced nor heated. (B) Same sample as that shown in panel A, but partially reduced with 10 mM DTT for 10 min at room temperature. (C) Superdex 200 gel filtration chromatogram for 50 μg of affinity-purified GPfib complexes (solid line). Furthermore, a column calibration with catalase (232 kDa), ferritin (440 kDa), thyroglobulin (669 kDa), and dextran blue (2,000 kDa) is shown (dotted line). OD 220nm, optical density at 220 nm; mAU, milli-adsorption units. (D and E) Fractions 11 to 17 of the gel filtration separation were resolved by 5% SDS-PAGE and analyzed by Western blotting. Samples were loaded either nondenatured (D) or partially reduced (E), and detection was carried out with KL25 (D) or WE 83.4 (E), respectively. Molecular mass markers (in kDa) are noted at the left of blots.