Figure 3.
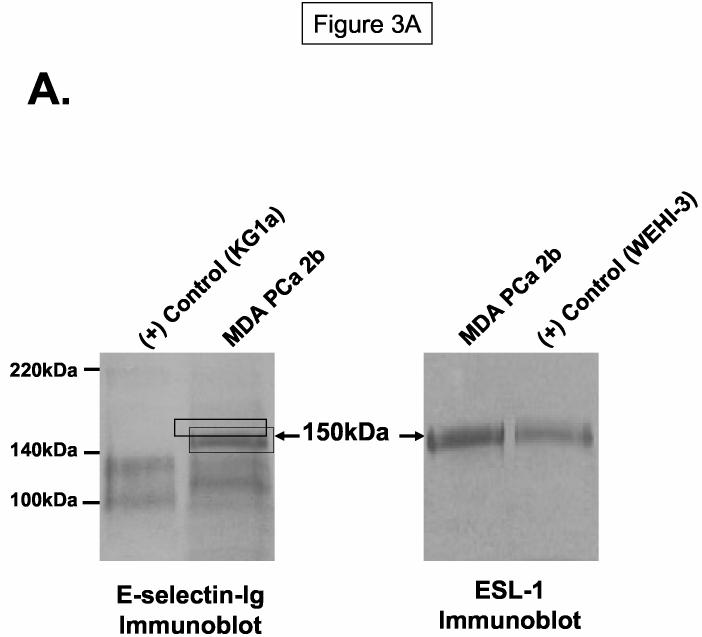
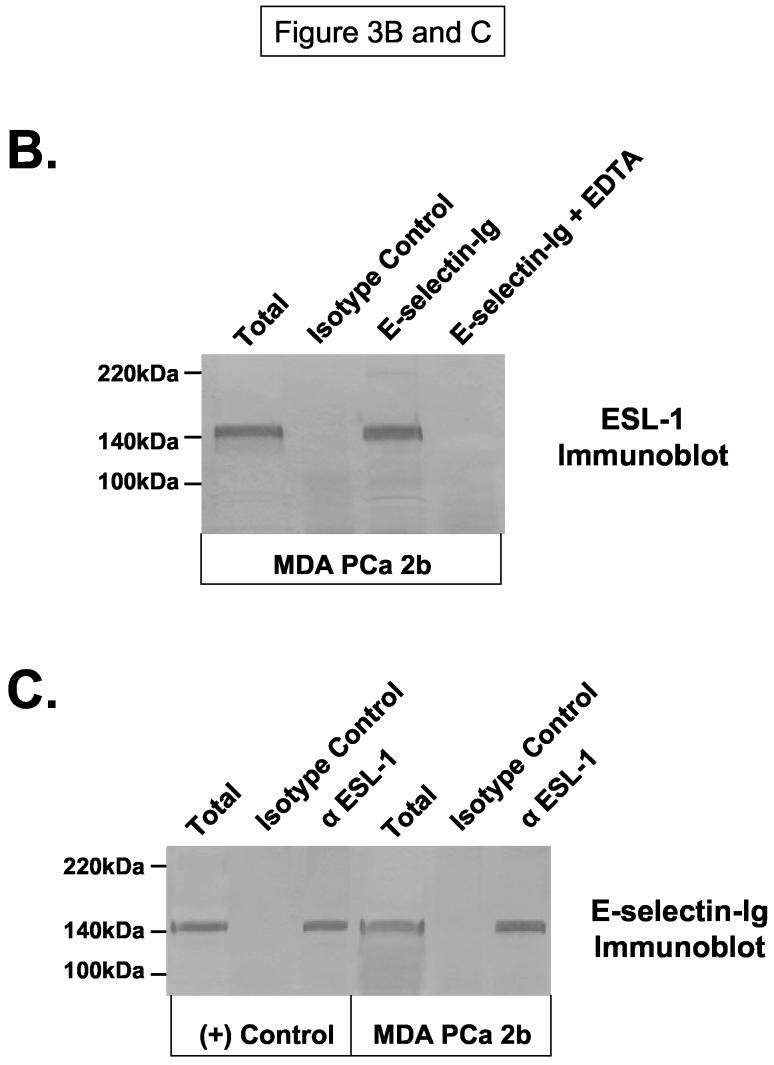
ESL-1 is a Candidate E-selectin Ligand on Human Bone-Metastatic Prostate Tumor Cells. Membrane protein and immunoprecipitates were resolved on 4-20% SDS-PAGE gels, transferred to PVDF membrane and blotted as indicated. In Panel A, ESL-1 was identified from the gel fragment (box) corresponding to the E-selectin-Ig-reactive membrane protein at 150kDa from MDA PCa 2b cells by mass spectrometry. Immunoblot analysis of (+) control WEHI-3 and of MDA PCa 2b membrane protein (40μg/Lane) with ESL-1 anti-sera showed a stainable protein at 150kDa. To determine whether ESL-1 was the E-selectin glycoprotein ligand at 150kDa, E-selectin-Ig affinity-purified eluate from MDA PCa 2b membrane protein (300μg) was blotted with ESL-1 anti-sera (Panel B). Also, anti-ESL-1 immunoprecipitates of MDA PCa 2b membrane protein (300μg) were immunoblotted with E-selectin-Ig (Panel C). In Panel B, the 150kDa protein was detected in E-selectin-Ig eluate with ESL-1 anti-sera and not in the E-selectin-Ig eluate containing EDTA. In Panel C, the 150kDa protein was blotted with E-selectin-Ig in membrane protein (40μg/Lane) and anti-ESL-1 immunoprecipitates from WEHI-3 and MDA PCa 2b cells. Immunoblots performed with AP-secondary Ab alone did not result in background staining. Immunoblots are representative of a minimum of 5 experiments.
