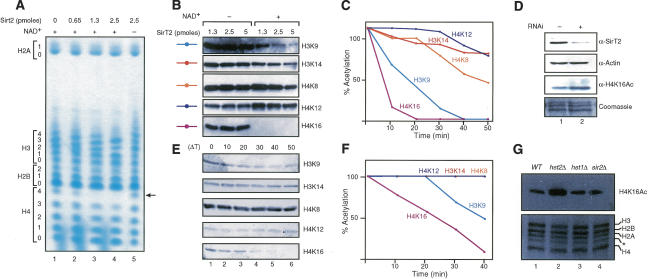
Figure 1.
SirT2 and Hst2p are NAD+-dependent histone deacetylases with a preference for H4K16Ac in vitro and in vivo. (A) TAU gel analysis of hyperacetylated core histones treated with the indicated amounts of SirT2 in the presence and absence of NAD+. Levels of acetylation for each core histone are indicated. (B) Hyperacetylated core histones were incubated with the indicated amounts of SirT2 in the presence and absence of NAD+ followed by Western blot using antibodies against specific acetylated residues in H3 and H4. (C) Time-course experiment of core histone deacetylation by SirT2 in the presence of NAD+ analyzed as in B. Quantifications were determined as indicated previously. (D) RNAi experiments against SirT2 in 293 cells. Whole-cell extracts were probed for the presence of SirT2 and actin by Western blot. Histones were extracted with hydrochloric acid and tested for H4K16Ac levels by Western blot. Levels of total histones were visualized with Coomassie blue staining. (E) Western blot of a time-course experiment for deacetylation performed as in C but with purified Hst2p. (F) Graph of the quantifications of E determined as in C. (G, top) Levels of H4K16Ac and total histones purified from isogenic S. cerevisiae strains (wild type, W303-1b; hst2Δ, YRH45; sir2Δ, YRH15; hst1Δ LNYY315) analyzed by Western blot. The bottom panel shows the histones from the various mutants, stained with Coomassie. The asterisk indicates an H3 breakdown product commonly found in histones purified from yeast (Edmondson et al. 1996).