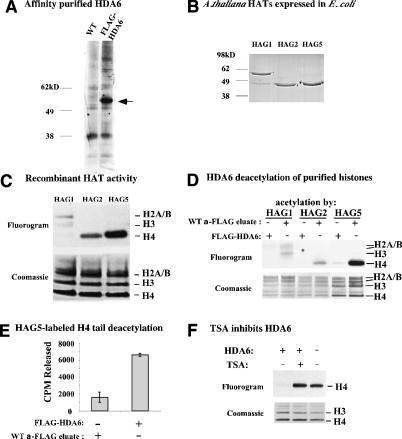
Figure 3.
Purified HDA6 has HDAC activity. (A) Affinity purification of Flag-HDA6 expressed in transgenic plants. Extracts of wild-type or Flag-HDA6-overexpressing A. thaliana was incubated with anti-Flag resin. A Coomassie blue-stained SDS-PAGE gel of proteins eluted using excess Flag peptide is shown. (B) Coomassie-stained SDS-PAGE gel of His-tagged recombinant Arabidopsis HATs HAG1, HAG2, and HAG5 after purification on nickel-agarose. (C) HAT activity of HAG1, HAG2, and HAG5. Broccoli histones were labeled using 3H-acetyl CoA and the resulting SDS-PAGE gel was Coomassie-stained and subsequently subjected to fluorography. Histone H3 and H4 bands were definitively identified using mass spectrometry. H2A and H2B variants with overlapping migration patterns precluded definitive assignment of H2A and H2B bands. (D) HDA6 deacetylates full-length histones acetylated by HAG1, HAG2, and HAG5. Histones labeled by the HATs were incubated with equal aliquots of Flag-HDA6 or wild-type protein eluted from anti-Flag resin. The fluorogram and Coomassie-stained histone bands are shown. (E) HDA6 deacetylates histone N-peptides. Anti-Flag resin incubated with extracts of wild-type or Flag-HDA6-expressing plants was washed extensively then incubated with HAG5-labeled H4 peptide immobilized on agarose beads. 3H (from 3H-acetyl CoA) released into the reaction buffer was measured by scintillation counting. (F) HDA6 is a TSA-sensitive HDAC. HAG5-labeled broccoli histones were incubated with HDA6 ± TSA and then subjected to SDS-PAGE and fluorography.