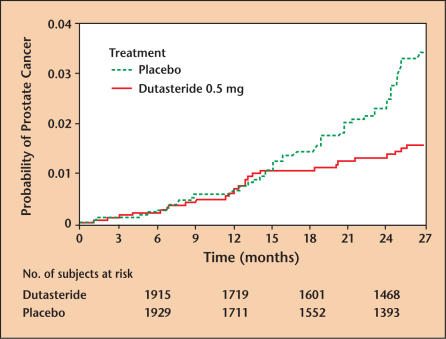
Figure 11.
Kaplan-Meier estimates of proportion of subjects experiencing a prostate cancer adverse event in the dutasteride phase III trial. Note that starting at approximately 15 months into the trial, the placebo and dutasteride groups begin to diverge. For the first 27 months, the cumulative incidence of CaP was 1.2% in the dutasteride group and 2.5% in the placebo group. The differences are highly significant (P = .002), suggesting a chemopreventive effect in the dutasteride group. CaP, cancer of the prostate. Reprinted with permission from Andriole GL et al.34